Abstract 摘要
Aims 研究目的
Plant growth-promoting bacteria (PGPB) affect host physiological processes in various ways. This study aims at elucidating the dependence of bacterial-induced growth promotion on the plant genotype and characterizing plant metabolic adaptations to PGPB.
促進植物生長細菌(PGPB)以多種方式影響宿主的生理過程。本研究旨在闡明細菌誘導的生長促進作用對植物基因型的依賴性,並描述植物對 PGPB 的代謝適應。
Methods 方法
Eighteen Arabidopsis thaliana accessions were inoculated with the PGPB strain Kosakonia radicincitans DSM 16656T. Colonisation pattern was assessed by enhanced green fluorescent protein (eGFP)-tagged K. radicincitans in three A. thaliana accessions differing in their growth response. Metabolic impact of bacterial colonisation was determined for the best responding accession by profiling distinct classes of plant secondary metabolites and root exudates.
將十八種阿拉伯芥(Arabidopsis thaliana)品系接種 PGPB 菌株 Kosakonia radicincitans DSM 16656 T 。透過增強型綠色螢光蛋白(eGFP)標記的 K. radicincitans,評估三種生長反應不同的阿拉伯芥品系中的定殖模式。針對反應最佳的品系,通過分析不同類別的植物次級代謝物和根系分泌物來確定細菌定殖的代謝影響。
Results 結果
Inoculation of 18 A. thaliana accessions resulted in a wide range of growth responses, from repression to enhancement. Testing the bacterial colonisation of three accessions did not reveal a differential pattern. Profiling of plant secondary metabolites showed a differential accumulation of glucosinolates, phenylpropanoids and carotenoids in roots. Analysis of root exudates demonstrated that primary and secondary metabolites were predominantly differentially depleted by bacterial inoculation.
接種 18 種阿拉伯芥(A. thaliana)種質後觀察到從抑制到促進的廣泛生長反應差異。針對其中三種種質進行細菌定殖測試並未顯示差異性模式。植物次級代謝物分析顯示根部葡萄糖苷酸酯、苯丙烷類化合物和類胡蘿蔔素的差異性累積。根系分泌物分析證實,經細菌接種後主要與次級代謝物均呈現顯著差異性耗竭現象。
Conclusions 結論
The plant genotype controls the bacterial growth promoting traits. Levels of lutein and β-carotene were elevated in inoculated roots. Supplementing a bacterial suspension with β-carotene increased bacterial growth, while this was not the case when lutein was applied, indicating that β-carotene could be a positive regulator of plant growth promotion.
植物基因型主導細菌促生長特性。接種後根部葉黃素與β-胡蘿蔔素含量顯著提升。當細菌懸浮液添加β-胡蘿蔔素時可促進細菌生長,但添加葉黃素則無此效應,顯示β-胡蘿蔔素可能作為植物生長促進的正向調控因子。
Similar content being viewed by others
其他使用者同時瀏覽的相似內容
Explore related subjects
探索相關主題
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.探索相關領域研究人員的最新文章與新聞,透過機器學習推薦。
Avoid common mistakes on your manuscript.
Introduction 緒論
Bacteria can colonize the plant rhizosphere, phyllosphere and reproductive organs of plants (Bodenhausen et al. 2013; Compant et al. 2010; Rosenblueth and Martinez-Romero 2006), with some, referred to as plant growth-promoting bacteria (PGPB), capable of stimulating the growth of the host, and others of increasing host fitness. PGPB are able to fix atmospheric nitrogen, enhance nutrient uptake (P, N, Mg, K) and modulate plant development via the modulation of phytohormone production (Berg 2009; Weyens et al. 2009). Biological atmospheric nitrogen fixation by diazotrophic bacteria is of enormous importance to agriculture, as it provides means to optimize nitrogen fertilisation regimes in agriculture and reduce environmental nitrogen pollution (Vessey 2003). Phosphate-solubilizing bacteria are widely distributed in the rhizosphere, operating via the secretion of organic acids and phosphatases in calcareous soils (Richardson and Simpson 2011), as are those which increase the availability of iron and phosphate by the excretion of siderophores or chelators in acidic soils (Matsuoka et al. 2013). A range of bacterial species is known to synthesize (or degrade) the key phytohormones abscisic acid, ethylene, gibberellins and indole-3-acetic acid (IAA) (Dodd et al. 2010). In most cases the presence of PGPB affects the development of primary and lateral roots of inoculated plants as a consequence of bacterial manipulation of host metabolism (El Zemrany et al. 2007; Verbon and Liberman 2016). The overall plant growth benefits from the modified root system architecture either directly by increased uptake of nutrients and water, or indirectly through biocontrol of phytopathogens (Lugtenberg and Kamilova 2009).
細菌能夠定殖於植物的根際圈、葉際圈及生殖器官(Bodenhausen 等人,2013;Compant 等人,2010;Rosenblueth 與 Martinez-Romero,2006),其中部分被稱為植物生長促進細菌(PGPB),能夠刺激宿主生長,另一些則能提升宿主適應性。PGPB 具有固定大氣氮素、促進養分吸收(磷、氮、鎂、鉀),以及透過調節植物荷爾蒙生產來調控植物發育的能力(Berg,2009;Weyens 等人,2009)。固氮細菌進行的生物大氣固氮作用對農業極具重要性,因其能優化農業氮肥施用策略並減少環境氮污染(Vessey,2003)。溶磷細菌廣泛分布於根際圈,在石灰質土壤中透過分泌有機酸與磷酸酶發揮作用(Richardson 與 Simpson,2011),而在酸性土壤中則透過分泌鐵載體或螯合劑來提高鐵與磷的有效性(Matsuoka 等人,2013)。 已知多種細菌物種能夠合成(或降解)關鍵植物激素,包括脫落酸、乙烯、赤黴素和吲哚-3-乙酸(IAA)(Dodd 等人,2010 年)。在多數情況下,植物生長促進細菌(PGPB)的存在會影響接種植物的初生根和側根發育,這是細菌調控宿主代謝的結果(El Zemrany 等人,2007 年;Verbon 和 Liberman,2016 年)。植物整體生長受益於改變後的根系結構,這種益處可能直接來自於營養與水分吸收能力的提升,或間接透過對植物病原菌的生物防治作用實現(Lugtenberg 和 Kamilova,2009 年)。
While the in vitro characterization of PGPB has provided insights into possible mechanisms underlying plant growth promotion, their mode of action in planta remains unclear. The impact on the Arabidopsis thaliana transcriptome following the plant’s colonization by Bacillus subtilis (Lakshmanan et al. 2013), Burkholderia phytofirmans (Poupin et al. 2013), Pseudomonas thivervalensis (Cartieaux et al. 2003), P. fluorescens strains (van de Mortel et al. 2012; Verhagen et al. 2004; Wang et al. 2005; Weston et al. 2012) and a Pseudomonas species (Schwachtje et al. 2011) has been described. While these PGPB induce changes in primary metabolism, defence-related pathways and hormone signalling, the details of the plant’s physiological response are strongly dependent on the interaction between the bacterium and its host plant. Recent reports describe the changes to the host metabolome provoked by PGPB (Berger et al. 2017; Vacheron et al. 2013). Especially the composition and concentration of plant secondary metabolites, such as glucosinolates, phenylpropanoids and carotenoids, is affected by the colonisation of bacteria, indicating that these compounds are involved in plant-bacteria interaction, although their function is largely unclear (Chamam et al. 2013; Ruppel et al. 2008; van de Mortel et al. 2012; Walker et al. 2011; Walker et al. 2012). Plant roots secrete constantly a variety of compounds into the rhizosphere as a part of the rhizodeposition process and to influence microbial communities in their immediate vicinity (Bressan et al. 2009). The role of some root secondary compounds in the communication between soil microbes and plants has been resolved. Flavonoids, a major class of phenylpropanoids, are known to induce the expression of nodulation genes in rhizobia for initiation of symbiosis in root exudates (Zhang et al. 2015). Degradation products of carotenoids, mycorradicin and strigolactones, are involved in the establishment of arbuscular mycorrhiza in the host root (Walter 2013). It has been largely recognized that the metabolite constitution of root exudates governs the recognition process between plants and microbes (Bertin et al. 2003; Faure et al. 2009). Hence, the concerted analysis of the metabolite composition of roots and root exudates is of utmost importance for understanding plant-bacteria interaction (Narula et al. 2009).
儘管 PGPB(植物生長促進細菌)的體外特性研究已為植物生長促進機制提供見解,但其在植物體內的作用模式仍不清楚。先前研究已描述阿拉伯芥(Arabidopsis thaliana)在受到枯草桿菌(Bacillus subtilis)(Lakshmanan 等人 2013)、伯克霍爾德菌(Burkholderia phytofirmans)(Poupin 等人 2013)、假單胞菌(Pseudomonas thivervalensis)(Cartieaux 等人 2003)、螢光假單胞菌(P. fluorescens)菌株(van de Mortel 等人 2012;Verhagen 等人 2004;Wang 等人 2005;Weston 等人 2012)及一種假單胞菌屬(Schwachtje 等人 2011)定殖後轉錄組的變化。雖然這些 PGPB 會誘導初級代謝、防禦相關路徑與激素信號的改變,但植物生理反應的細節高度取決於細菌與宿主植物間的交互作用。近期研究報告描述了 PGPB 引發的宿主代謝組變化(Berger 等人 2017;Vacheron 等人 2013)。 特別是植物次級代謝產物的組成與濃度,如硫代葡萄糖苷、苯丙烷類化合物和類胡蘿蔔素,會受到細菌定殖的影響,這表明這些化合物參與了植物與細菌的相互作用,儘管其功能尚不清楚(Chamam 等人,2013;Ruppel 等人,2008;van de Mortel 等人,2012;Walker 等人,2011;Walker 等人,2012)。植物根部作為根際沉積過程的一部分,會持續分泌多種化合物至根際,以影響其周圍的微生物群落(Bressan 等人,2009)。某些根部次級化合物在土壤微生物與植物之間的通訊作用已被解析。黃酮類化合物作為苯丙烷類化合物的主要類別,已知可誘導根瘤菌中結瘤基因的表達,以啟動根部分泌物中的共生關係(Zhang 等人,2015)。類胡蘿蔔素的降解產物,如菌根素和獨腳金內酯,則參與宿主根部叢枝菌根的建立(Walter,2013)。 已有廣泛研究證實,根系分泌物的代謝物組成主導了植物與微生物之間的識別過程(Bertin 等人,2003;Faure 等人,2009)。因此,同步分析根部與根系分泌物的代謝物組成,對於理解植物-細菌相互作用至關重要(Narula 等人,2009)。
Presence of the gram negative bacterium Kosakonia radicincitans DSM 16656T (syn. Enterobacter radicincitans (Brady et al. 2013), formerly Pantoea agglomerans) in the phyllosphere of winter wheat has been described previously (Kämpfer et al. 2005; Ruppel 1988). It has been shown that the inoculation of this strain stimulates root and shoot growth in a range of plant hosts (Berger et al. 2015; Brock et al. 2013; Höflich and Ruppel 1994; Schreiner et al. 2009). These bacteria, at least in vitro, are capable of both nitrogen fixation and phosphorus solubilization (Ruppel and Merbach 1995; Schilling et al. 1998). A possible involvement in the host’s phytohormone status has been inferred by their ability to synthesize IAA and the cytokinins N6-isopentenyl-adenosine and -adenine (Scholz-Seidel and Ruppel 1992). The growth-promoting properties of K. radicincitans were demonstrated for a wide range of hosts but the degree of plant beneficial effects is determined by the plant species and genotype (Remus et al. 2000; Schreiner et al. 2009).
先前研究已記載革蘭氏陰性菌 Kosakonia radicincitans DSM 16656 T (同義名:Enterobacter radicincitans (Brady 等人 2013 年),舊稱 Pantoea agglomerans)存在於冬小麥的葉際中(Kämpfer 等人 2005 年;Ruppel 1988 年)。研究顯示接種此菌株能促進多種植物宿主的根與莖生長(Berger 等人 2015 年;Brock 等人 2013 年;Höflich 與 Ruppel 1994 年;Schreiner 等人 2009 年)。該細菌至少於體外實驗中,同時具有固氮與溶磷能力(Ruppel 與 Merbach 1995 年;Schilling 等人 1998 年)。透過其合成 IAA 及細胞分裂素 N6-異戊烯基腺苷與腺嘌呤的能力(Scholz-Seidel 與 Ruppel 1992 年),可推測其可能參與宿主植物激素調節。K. radicincitans 的促生長特性在廣泛宿主中皆獲證實,但植物受益程度取決於物種與基因型(Remus 等人 2000 年;Schreiner 等人 2009 年)。
The present study focused on characterizing the impact of K. radicincitans on plant metabolism when colonizing A. thaliana with special emphasis on glucosinolates, phenylpropanoids and carotenoids. We demonstrate that, as a consequence of the bacterial-induced alterations in the root metabolite profile, the root exudate pattern is also greatly affected by the presence of the bacterium. Moreover, we found evidence that the presence of K. radicincitans, as visualized by enhanced green fluorescent protein (eGFP)-tagged bacteria, did not correlate with the level of growth promotion when multiple plant accessions were tested.
本研究重點在於探討 K. radicincitans 定殖於阿拉伯芥時對植物代謝的影響,特別聚焦於硫代葡萄糖苷、苯丙烷類化合物及類胡蘿蔔素。我們證實由於細菌誘導的根部代謝物譜改變,根系分泌模式亦顯著受到該菌存在的影響。此外,透過增強型綠色螢光蛋白(eGFP)標記菌株觀察發現,當測試多種植物種源時,K. radicincitans 的存在與生長促進程度並無顯著相關性。
Materials and methods 材料與方法
Plant material and cultivation
植物材料與栽培
A collection of 18 A. thaliana accessions (Bur-0, Can-0, Col-0, Ct-1, Edi-0, Hi-0, Kn-0, Ler-0, Mt.-0, No-0, Oy-0, Po-0, Rsch-4, Sf-2, Tsu-0, Wil-2, Ws-0, Wu-0, kindly provided by L. Westphal, Leibniz Institute of Plant Biochemistry, Germany, Supplemental Fig. S1) was used to assess the growth-promoting effects of K. radicincitans. Host plants were raised on non-sterile standard plant growth substrate (Fruhstorfer Erde type P, Germany) under short day conditions (8 h photoperiod) at 22 °C and 40–60% relative humidity. Two week old seedlings were individually potted into sand for inoculation with K. radicincitans strain DSM 16656T. The accession screen was performed once using 20 plants per accession and treatment. Accession Oy-0 grown for plant secondary metabolite profiling and root exudate collection was cultivated as described above. The pots were watered with nutrient solution as described by Gibeaut et al. (1997), and after four weeks of growth, the material was harvested. Roots were washed to remove adhering sand particles and blotted dry on tissue paper. Rosette leaves and roots were separately snap-frozen in liquid nitrogen. These experiments were performed in triplicate using 20–25 plants per treatment that were pooled into one sample.
本研究使用 18 種阿拉伯芥(Arabidopsis thaliana)生態型(Bur-0、Can-0、Col-0、Ct-1、Edi-0、Hi-0、Kn-0、Ler-0、Mt.-0、No-0、Oy-0、Po-0、Rsch-4、Sf-2、Tsu-0、Wil-2、Ws-0、Wu-0,由德國萊布尼茲植物生化研究所 L. Westphal 博士惠贈,補充圖 S1)來評估放射根科薩克尼亞菌(Kosakonia radicincitans)的促生長效應。宿主植物栽培於非無菌標準植物生長基質(德國 Fruhstorfer Erde type P)中,生長條件為短日照(8 小時光周期)、22°C 及 40-60%相對濕度。兩週齡幼苗分別移植至沙質基質中進行 K. radicincitans 菌株 DSM 16656 接種。每種生態型與處理組合使用 20 株植物進行單次篩選試驗。用於植物次級代謝物分析與根系分泌物收集的 Oy-0 生態型,其栽培條件同上。盆栽使用 Gibeaut 等人(1997)所述營養液進行澆灌,生長四周後採收材料。根部經清洗去除附著沙粒後以濾紙吸乾水分,蓮座葉與根部分別以液態氮快速冷凍保存。 這些實驗均以三重複進行,每個處理使用 20-25 株植物,並將其混合為一個樣本。
Bacterial colonisation was quantified in gtr1gtr2 (At3g47960, At5g62680), sds1 (At1g78510.1), ccoaomt1 (At4g34050) and f6’h1 (At3g13610) knock-out mutants of A. thaliana. Here, four-week-old plants were grown as described above and inoculated with K. radicincitans. Roots were harvested after 4 days. Three batches of plants were analysed, consisting of 10 plants each.
在阿拉伯芥(Arabidopsis thaliana)的 gtr1gtr2(At3g47960、At5g62680)、sds1(At1g78510.1)、ccoaomt1(At4g34050)和 f6’h1(At3g13610)基因敲除突變株中,對細菌定殖進行了定量分析。實驗採用四週齡植株,按上述方法培養並接種 K. radicincitans 菌株。接種 4 天後採收根部樣本。每批次分析 10 株植物,共進行三個批次的實驗。
Accessions Col-0, Ler-0 and Oy-0 grown for in situ localisation studies were raised as in vitro cultures. Seeds were surface-sterilized with a solution containing 5% NaOCl and 0.5% Tween 20 and then incubated at 4 °C for 3 days for stratification. Seeds were sown on sterile plates containing 1/2-strength Murashige-Skoog medium supplemented with 1.5% sucrose and grown as described above under short day conditions. After one week, germinated plants were transferred to square plates with the same medium and kept in a vertical position for further three weeks.
用於原位定位研究的 Col-0、Ler-0 和 Oy-0 種系以離體培養方式培育。種子先以含 5%次氯酸鈉和 0.5% Tween 20 的溶液進行表面滅菌,接著在 4°C 下層積處理 3 天。將種子播種於含 1/2 濃度 Murashige-Skoog 培養基(添加 1.5%蔗糖)的無菌培養皿中,並按前述方法在短日照條件下培育。一週後,將發芽植株移植至相同培養基的方形培養皿中,並保持垂直位置繼續培養三週。
Bacteria cultivation, transformation and plant inoculation
細菌培養、轉型與植物接種
The bacteria were cultured overnight in standard nutrient broth (Merck, Germany) according to Ruppel et al. (2006). The cells were first pelleted by centrifugation, washed twice in sterile 50 mM NaCl, re-suspended in 50 mM NaCl to give an OD620 of 0.2 (corresponding to 109 cfu mL−1) and finally further diluted to a concentration of 107 cfu mL−1. Either a 10 mL aliquot of this cell suspension or 10 mL 50 mM NaCl as control treatment was spread over the surface of each pot.
根據 Ruppel 等人(2006)的方法,將細菌於標準營養肉湯(Merck,德國)中培養過夜。首先通過離心沉澱細胞,用無菌 50 mM NaCl 洗滌兩次,重新懸浮於 50 mM NaCl 中使 OD 值達 0.2(相當於 10^1 cfu/mL),最後進一步稀釋至 10^3 cfu/mL 濃度。每盆表面均勻塗布 10 mL 此細胞懸浮液或 10 mL 50 mM NaCl(作為對照處理)。
For in situ localisation studies, electro competent bacterial cells were transformed with plasmid pMP4655 (Bloemberg et al. 2000; Lagendijk et al. 2010). Single colonies of K. radicincitans expressing eGFP grown on Luria-Bertani agar plus gentamycin (150 μg/ml) were inoculated in 50 ml standard nutrient broth and preparation of inoculation suspension was as described above. Plants were carefully removed from agar plates, the roots gently soaked in water in a dish and transferred into 12 ml tubes containing 11 ml sterile water. A. thaliana plants were left for 24 h to adjust to the changed conditions before 105 bacterial cells were added to each plant in 11 ml sterile water. Water levels were adjusted on a daily basis to account for evaporation and plant-based water losses.
針對原位定位研究,我們使用電穿孔感受態細菌細胞轉化質體 pMP4655(Bloemberg 等人,2000;Lagendijk 等人,2010)。將表現 eGFP 的 K. radicincitans 單一菌落(培養於含慶大黴素 150 μg/ml 的 Luria-Bertani 瓊脂培養基)接種至 50 毫升標準營養肉湯中,接種懸浮液製備方法如前述。小心將植物從瓊脂培養皿取出,根部輕柔浸泡於培養皿的水中後轉移至含 11 毫升無菌水的 12 毫升試管。阿拉伯芥植株在添加每株 10 5 個細菌細胞(懸浮於 11 毫升無菌水)前,先靜置 24 小時以適應環境變化。每日調整水位以補償蒸發及植物水分消耗。
Confocal laser scanning microscopy (CLSM)
共軛焦雷射掃描顯微鏡(CLSM)
Bacterial root colonization was monitored after 24 h, 48 h and 6 days. Roots were gently washed in sterile water and fluorescence was recorded with a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss Jena GmbH, Germany). Bacterial eGFP fluorescence signals were captured by confocal laser microscopy on a Zeiss LSM 510 META confocal system (excitation/emission 488 nm, samples at 8% argonlaser power, BP505–550 filter, Plan Apo 63/1.4 oil lens) and roots were captured using bright field settings.
在 24 小時、48 小時和 6 天後監測細菌根部定殖情況。根部以無菌水輕柔沖洗後,使用蔡司 LSM 510 META 雷射掃描共軛焦顯微鏡(德國 Carl Zeiss Jena GmbH 公司)記錄螢光訊號。細菌 eGFP 螢光訊號透過蔡司 LSM 510 META 共軛焦系統(激發/發射波長 488 nm,樣品使用 8%氬雷射功率,BP505–550 濾鏡,Plan Apo 63/1.4 油鏡)進行共軛焦雷射顯微鏡拍攝,根部影像則採用明視野設定拍攝。
Detection of K. radicincitans in planta using qPCR
使用 qPCR 檢測植物體內 K. radicincitans 的存在
K. radicincitans specific primer design
K. radicincitans 特異性引子設計
Comparing the genome of DSM 16656T (Witzel et al. 2012) to genomes of other Enterobacteriaceae and more distantly related bacteria, a DNA repair protein was found to be specific to Kosakonia radicincitans and closely related taxa. NCBI blastn searches confirmed the specificity of this gene in silico (Supplemental Table S1). DNA sequences of DSM 16656 T and close relatives were aligned in order to determine highly variable sites (Supplemental Fig. S2). K. radicincitans specific primers were designed using Geneious (http://www.geneious.com/) from Biomatters and Oligo Calc (http://biotools.nubic.northwestern.edu/OligoCalc.html) from Northwestern University.
將 DSM 16656 T (Witzel et al. 2012)的基因組與其他腸桿菌科及親緣關係較遠的細菌基因組進行比較後,發現一種 DNA 修復蛋白為 Kosakonia radicincitans 及其近緣分類群所特有。NCBI blastn 搜尋結果在電腦模擬中證實了該基因的特異性(補充表 S1)。為確定高度變異位點,我們對 DSM 16656 T 及其近緣種的 DNA 序列進行了比對(補充圖 S2)。K. radicincitans 特異性引子是使用 Biomatters 公司的 Geneious 軟體(http://www.geneious.com/)與西北大學的 Oligo Calc 工具(http://biotools.nubic.northwestern.edu/OligoCalc.html)所設計。
Bacterial colonization studies
細菌定殖研究
Root colonization of Col-0 ecotype and gtr1gtr2, sps1, ccoaomt1 and f6’h1 knock-out mutants of A. thaliana by K. radicincitans was determined by qPCR. Genomic DNA was extracted using DNeasy Plant Kit from Qiagen (Düren, Germany) following the manufacturer’s instructions. DNA quantity and quality were assessed with the NanoDrop (Thermo Scientific, Bonn, Germany) system. The qPCRs with strain specific primers were performed using primers fdnaJ_F1 (5`-AAGCCAGCGTTCCGTCGTA-3`) and fdnaJ_R2 (5`-GATCGTTGAACTCGTCGAGCAG-3`) with a product size of 140 bp. Approximately 10 ng of genomic plant DNA was used for each qPCR with SsoAdvanced Universal SYBR Green Supermix (BioRad, Germany). The PCR conditions were as follow: Initially 3 min 95 °C, then 35 cycles of 95 °C for15 sec, followed by 50 s at 72 °C and 5 min of elongation time at 72 °C. Each of the biological replicates was analyzed in three technical replications. Two reference genes (At5g55840, At5g08290) (Witzel et al. 2013) were used for relative quantification (Livak and Schmittgen 2001) of K. radicincitans in A. thaliana roots. qPCR melting curve analysis was performed to ensure the presence of a single product.
利用定量聚合酶鏈式反應(qPCR)測定 K. radicincitans 對阿拉伯芥 Col-0 生態型及 gtr1gtr2、sps1、ccoaomt1 與 f6'h1 基因剔除突變株的根部定殖情況。基因組 DNA 使用 Qiagen 公司(德國杜倫)的 DNeasy Plant Kit 依照製造商說明進行萃取。DNA 濃度與品質透過 NanoDrop 系統(賽默飛世爾科技,德國波昂)進行評估。採用菌株特異性引子 fdnaJ_F1 (5'-AAGCCAGCGTTCCGTCGTA-3')與 fdnaJ_R2 (5'-GATCGTTGAACTCGTCGAGCAG-3')進行 qPCR,產物大小為 140 bp。每次反應使用約 10 ng 植物基因組 DNA,搭配 SsoAdvanced Universal SYBR Green Supermix(德國 BioRad)。PCR 條件如下:初始 95°C 3 分鐘,隨後進行 35 個循環(95°C 15 秒→72°C 50 秒),最後 72°C 延伸 5 分鐘。每個生物重複樣本進行三次技術重複分析。採用兩個參考基因(At5g55840、At5g08290)(Witzel 等人 2013 年)對阿拉伯芥根部 K. radicincitans 進行相對定量分析(Livak 與 Schmittgen 2001 年)。透過 qPCR 熔解曲線分析確認單一產物存在。
Root secondary metabolite profiling
根系次級代謝物分析
Glucosinolate analysis 硫代葡萄糖苷分析
Desulfo-glucosinolate profiles and concentrations were determined as described by Witzel et al. (2013).
脫硫硫代葡萄糖苷的組成與濃度測定係依據 Witzel 等人(2013)所述方法進行。
Flavonoid determination 類黃酮測定
Homogenized frozen root (100 mg) material were each extracted in 600 μL 60% methanol, shaken for 60 min at 20 °C and centrifuged (10,000 g for 10 min). The pellet was then re-extracted, first in 400 μL 60% methanol for 20 min and then in 200 μL 60% methanol for 10 min. The combined supernatants were filtered through a Spin-X tube (Sigma Aldrich, United States) by centrifugation (1000 g for 5 min) and dried by vacuum centrifugation. The residue was dissolved in 200 μL distilled water. Flavonoid glycosides and hydroxycinnamic acid derivatives were detected using HPLC-DAD-ESI-MSn as described by Neugart et al. (2012), with some modification to the solvent gradient. Specifically, solvent A consisted of 99.5% water, 0.5% acetic acid and solvent B of 100% acetonitrile. The sequence was as follows: 0–12 min: linear increase of B from 5% to 7%; 12–15 min: linear increase of B from 7% to 9%; 15–45 min: linear increase of B from 9% to 12%; 45–100 min: linear increase of B from 12% to 15%; 100–105 min: linear increase of B from 15% to 75%; 105–115 min: 75% B; 115–120 min: linear decrease of B from 75% to 5%; 120–123 min: 5% B. The flow rate was 0.4 mL min−1, and the detector wavelengths were 320 nm for the hydroxycinnamic acid derivatives and 370 nm for the flavonol glycosides. These were both identified as deprotonated molecular ions and characteristic mass fragment ions by HPLC-DAD-ESI-MSn using an Agilent series 1100 ion trap mass spectrometer run in negative ionization mode.
將 100 毫克均質冷凍根部樣品分別以 600 微升 60%甲醇萃取,於 20°C 震盪 60 分鐘後離心(10,000 g,10 分鐘)。殘渣先以 400 微升 60%甲醇再萃取 20 分鐘,再以 200 微升 60%甲醇萃取 10 分鐘。合併上清液後,使用 Spin-X 離心管(美國 Sigma Aldrich)以 1000 g 離心 5 分鐘過濾,並經真空離心乾燥。殘留物以 200 微升蒸餾水溶解。類黃酮苷與羥基肉桂酸衍生物之檢測採用 HPLC-DAD-ESI-MS n 系統,參考 Neugart 等人(2012)方法並修改溶劑梯度:溶劑 A 為 99.5%水與 0.5%醋酸,溶劑 B 為 100%乙腈。梯度程序如下:0-12 分鐘:B 相由 5%線性升至 7%;12-15 分鐘:7%升至 9%;15-45 分鐘:9%升至 12%;45-100 分鐘:12%升至 15%;100-105 分鐘:15%升至 75%;105-115 分鐘:維持 75% B 相;115-120 分鐘:75%降至 5%;120-123 分鐘:維持 5% B 相。 流速為 0.4 mL/min,檢測器波長設定為:羥基肉桂酸衍生物 320 nm,黃酮醇苷類 370 nm。透過配備安捷倫 1100 系列離子阱質譜儀的 HPLC-DAD-ESI-MS 系統(於負離子模式下運行),這些化合物皆被鑑定為去質子化分子離子及特徵性質量碎片離子[2]。
Carotenoid determination 類胡蘿蔔素測定
Carotenoids were obtained by extracting 100 mg powdered root in chloroform (Baldermann et al. 2010), and detected using an Agilent Technologies 1290 Infinity UPLC device coupled with an Agilent Technologies 6230 TOF LC/MS system. The separation was performed in gradient mode (solvent A was 81:15:4 methanol:methyltert-butyl-ether:water, and solvent B: 6:90:4) on a C30 column (YMC Co. Ltd., Japan, YMC C30, 100 × 2.1 mm, 3 μm) at a flow rate of 0.2 mL min−1. To enhance ionization, 20 mM ammonium acetate was added to the mobile phase. Identification was achieved by co-chromatography with reference compounds, and quantification from a dose response curve applying the following standards: neoxanthin [M-H2O + H]+ 583.415, lutein [M-H2O + H]+ 551.425, zeaxanthin [M + H]+ 569.435 and β-carotene [M + H]+ 537.446.
類胡蘿蔔素的提取方法是將 100 毫克根部粉末以氯仿進行萃取(Baldermann 等人 2010 年研究),並使用安捷倫科技 1290 Infinity 超高效液相層析儀串聯安捷倫科技 6230 飛行時間液質聯用系統進行檢測。分離過程採用梯度模式(溶劑 A 為甲醇:甲基叔丁基醚:水=81:15:4,溶劑 B 為 6:90:4),在 C 30 管柱(日本 YMC 公司 YMC C30,100×2.1 毫米,3 微米)上以 0.2 毫升/分鐘 −1 流速進行。為增強離子化效果,流動相中添加了 20 毫摩爾醋酸銨。通過與標準品共層析進行定性,並依據劑量反應曲線以下列標準品進行定量:新黃質[M-H 2 O+H] + 583.415、葉黃素[M-H 2 O+H] + 551.425、玉米黃質[M+H] + 569.435 及β-胡蘿蔔素[M+H] + 537.446。
Primary and secondary metabolite analysis of root exudates
根系分泌物初級與次級代謝物分析
Collection of root exudates was performed as described earlier with some modifications (Xu et al. 2016). In short, roots of plants either inoculated or non-inoculated with K. radicincitans were washed to remove any adhering sand and immersed in sterile distilled water for 1 h, and then transferred to a fresh batch of bi-distilled water for a further 4 h. The medium was filtered through a mixed cellulose ester membrane filter (pore size 0.22 μm, Carl Roth, Germany) to remove any cellular debris and external microorganisms, and concentrated tenfold by freeze-drying. The exudate from approximately 25 plants was pooled into a single sample. After collecting the exudate, the roots were weighed. Primary and secondary plant metabolite profiling was performed using GC-MS and LC-MS, respectively, on three replicate samples from three independent biological experiments.
根系分泌物的收集方法參考先前文獻並稍作修改(Xu et al. 2016)。簡而言之,將接種與未接種 K. radicincitans 的植株根部洗淨去除附著砂粒後,浸入無菌蒸餾水中 1 小時,再轉移至新鮮的雙蒸餾水中繼續收集 4 小時。收集液經混合纖維素酯膜過濾器(孔徑 0.22μm,德國 Carl Roth)過濾去除細胞碎片與外源微生物後,通過冷凍乾燥濃縮十倍。約 25 株植物的分泌物混合為單一樣本,採集後稱取根部鮮重。初級與次級植物代謝物分析分別採用 GC-MS 與 LC-MS 技術,每個處理設置三個重複樣本,共進行三次獨立生物學實驗。
For primary metabolite profiling, freeze-dried root exudates were derivatized and analysed by means of a GC-EI-Q-MS system as described previously (Strehmel et al. 2016). For secondary metabolite profiling, freeze-dried root exudates were reconstituted in 60 μL 30% methanol and analysed by UPLC-ESI-QTOF-MS system (Strehmel et al. 2014). All parameters were maintained as already described.
針對初級代謝物分析,冷凍乾燥的根系分泌物經衍生化處理後,採用 GC-EI-Q-MS 系統進行分析(方法參見 Strehmel 等人 2016 年文獻)。次級代謝物分析則將冷凍乾燥根系分泌物以 60 微升 30%甲醇重新溶解後,使用 UPLC-ESI-QTOF-MS 系統進行檢測(方法參見 Strehmel 等人 2014 年文獻)。所有分析參數均維持與前述文獻記載相同之設定。
In vitro bacterial growth assay in the presence of plant secondary metabolites
植物次級代謝物存在下的體外細菌生長試驗
Overnight cultures of K. radicincitans were diluted to 105 cfu/mL and grown in the presence or absence of plant secondary metabolites in standard nutrient broth for 15 h at 30 °C and 90 rpm. 2-Propenyl glucosinolate was dissolved in water and diluted to concentrations of 20, 40 and 100 μM, the carotenoids lutein and β–carotene were dissolved in Tween20 (1 g in 10 mL H20) and diluted to concentrations of 20, 40 and 100 μM, while phenylpronanoids (scopoletin, sinapic acid) and terpenes (squalene, α-humulene, farnesene) were all dissolved in 70% ethanol, each diluted to concentrations of 20 and 40 μM. Control samples of K. radicincitans received the same amount of water, Tween20 and 70% ethanol but without the plant metabolites. Each treatment was run in duplicate and the experiment was performed twice.
將 K. radicincitans 的過夜培養物稀釋至 10 5 CFU/mL,於標準營養肉湯中添加或不添加植物次級代謝產物,在 30°C、90 rpm 條件下培養 15 小時。2-丙烯基硫代葡萄糖苷溶於水並稀釋至 20、40 和 100 μM 濃度,類胡蘿蔔素葉黃素和β-胡蘿蔔素溶於 Tween20(1 克溶於 10 毫升 H 2 0)並稀釋至 20、40 和 100 μM 濃度,而苯丙素類(東莨菪亭、芥子酸)和萜類(角鯊烯、α-葎草烯、法尼烯)均溶於 70%乙醇,各稀釋至 20 和 40 μM 濃度。K. radicincitans 的對照組樣本添加等量水、Tween20 和 70%乙醇但不含植物代謝物。每種處理重複兩次,實驗進行兩次。
Statistical analyses 統計分析
Genotype and treatment effects on rosette biomass were tested using a two-way ANOVA (Holm-Sidak, SigmaPlot v12.3 software, Systat Software, Germany). The scatterplot was generated using SigmaPlot.
利用雙向變異數分析(Holm-Sidak 法,SigmaPlot v12.3 軟體,Systat Software,德國)檢測基因型和處理對蓮座葉生物量的影響。散點圖使用 SigmaPlot 生成。
Statistical evaluation of rosette biomass accumulation was performed using one-way ANOVA on ranks (Kruskal-Wallis) to detect differences in the mean values among groups of treated and non-treated plants (SigmaPlot). To identify the groups that differ from the other, normality testing (Shapiro-Wilk), equal variance test, followed by a two-tailed t-test was performed (SigmaPlot). Statistical testing of metabolite measurements and in vitro growth experiments was performed using a one-way ANOVA and a two-tailed Student’s t-test (SigmaPlot).
蓮座葉生物量累積的統計評估採用單因子等級變異數分析(Kruskal-Wallis 法)以檢測處理組與未處理組植物平均值間的差異(SigmaPlot)。為識別各組間的差異,先進行常態性檢定(Shapiro-Wilk 法)、等變異數檢定,再執行雙尾 t 檢定(SigmaPlot)。代謝物測量與體外生長實驗的統計檢驗採用單因子變異數分析與雙尾 Student t 檢定(SigmaPlot)。
Results 結果
Variation in the growth response of A. thaliana upon K. radicincitans inoculation
阿拉伯芥接種 K. radicincitans 後生長反應的變異
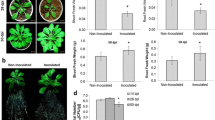
In order to survey the natural genetic variation of A. thaliana response for growth-promotion provoked by K. radicincitans, eighteen accessions were tested for alterations in rosette fresh weight four weeks after bacterial inoculation (Fig. 1a). The variation of biomass accumulation among the accessions ranged from 70% in Ler-0 to 140% in Oy-0, when compared to the non-inoculated plants of the same accession (set at 100%), demonstrating an extensive range of plant responses to the bacterium. Two-way ANOVA testing for genotype-by-treatment interaction revealed a significant effect of the genotype (p < 0.001) on rosette weight, while the effect of bacterial inoculation was lower (p = 0.447). In turn, there was a significant genotype-by-treatment interaction (p < 0.001). Regression analysis revealed a correlation between the fresh weight of control and inoculated plants, while growth promotion was not dependent on the genotype’s plant biomass production (Fig. 1b). Additional pairwise statistical analysis (Student’s t-tests), subsequent to one-way ANOVA (p < 0.001), comparing each accession with and without bacterial inoculation identified accessions with significant alterations in rosette biomass upon treatment (Supplemental Table S2).
為調查阿拉伯芥(A. thaliana)對 K. radicincitans 促生長作用之自然遺傳變異,本研究測試了 18 個生態型在接菌 4 週後蓮座葉鮮重之變化(圖 1a)。相較於未接菌之同生態型對照組(設定為 100%),各生態型生物量累積變異幅度介於 Ler-0 的 70%至 Oy-0 的 140%,顯示植物對該菌株之反應存在廣泛差異。雙因子變異數分析顯示基因型對蓮座葉重量具極顯著影響(p < 0.001),而接菌處理影響較小(p = 0.447);此外,基因型與處理間存在顯著交互作用(p < 0.001)。迴歸分析顯示接菌組與對照組鮮重具相關性,但促生長效果與生態型之生物量生產能力無關(圖 1b)。 在單因子變異數分析(p < 0.001)後進行的成對統計分析(Student's t 檢定),比較各品系接種與未接種細菌的差異,鑑定出處理後蓮座狀生物量有顯著變化的品系(補充表 S2)。
Relative growth of A. thaliana accessions grown in the presence or absence of K. radicincitans. The mean rosette fresh weight of inoculated plants (n = 20) was normalized to the same of control plants of the respective accession. Error bars indicate the standard error; capital letters denote significant difference between inoculated genotypes (p < 0.05, t-test). The dashed line indicates the 1:1 ratio between control and inoculated plants (a). Scatterplot comparison of the rosette fresh weight (non-normalized) obtained from inoculated or control plants produced a correlation coefficient of 0.911 (b)
在接種與未接種 K. radicincitans 情況下,阿拉伯芥各品系之相對生長量。接種植株(n=20)的平均蓮座葉鮮重已針對各品系對照組進行標準化處理。誤差線表示標準誤;大寫字母標示接種基因型間具顯著差異(p < 0.05,t 檢定)。虛線表示接種組與對照組間 1:1 比例關係(圖 a)。接種組與對照組蓮座葉鮮重(未經標準化)之散點圖比較顯示相關係數達 0.911(圖 b)
K. radicincitans colonizes the root surface of A. thaliana
K. radicincitans 定殖於阿拉伯芥根部表面
To probe whether the range of growth promotion observed in A. thaliana accessions was related to bacterial colonisation density, pattern of root colonisation were investigated using eGFP-transformed K. radicincitans and CLSM. The assay was performed using Oy-0 and Ler-0, representing the most responsive accessions (see Fig. 1), as well as Col-0 where previous testing was done (Brock et al. 2013). Attachment of bacteria onto the root surface and at root hairs was observed after 24 h (Fig. 2a) and adherence was tightly to the plant surface since cells were not washed off during the preparation procedure and were immobile. Bacteria formed a biofilm on the root surface at 48 h post inoculation (hpi) which consisted of only one or two cell layers. Bacterial numbers at individual colonization sites differed from several hundreds to only a few (Fig. 2b, c). The root cap region was not colonized. At 48 hpi bacteria were abundant at emerging lateral roots. At this position bacteria eventually enter the cells close to the junction where lateral root primordia push through the endodermis, the cortex and the epidermis. Colonization of lateral roots was rather sparse and restricted to the lateral root cracks (Fig. 2d, e). Rarely, single plant root cells were densely colonized by bacteria (Fig. 2f). No differences in root colonisation pattern were observed for Oy-0, Ler-0 and Col-0 at 24 hpi, 48 hpi and 6 dpi indicating that growth-promotion may be determined by the plant’s genotype specific response to colonisation, rather than actively by the bacterium.
為探究阿拉伯芥不同品系間觀察到的生長促進效果差異是否與細菌定殖密度有關,我們使用 eGFP 標記的 K. radicincitans 菌株與共軛焦顯微鏡(CLSM)觀察根部定殖模式。實驗選用反應最顯著的 Oy-0 與 Ler-0 品系(參見圖 1),以及先前研究使用過的 Col-0 品系(Brock 等人 2013 年研究)。接種 24 小時後可觀察到細菌附著於根表與根毛處(圖 2a),由於製備過程中未被沖洗脫落且保持固定狀態,顯示細菌與植物表面緊密黏附。接種後 48 小時(hpi),細菌在根表形成僅由 1-2 層細胞組成的生物膜。各定殖位點的細菌數量從數百個到僅少數不等(圖 2b、c)。根冠區域未發現定殖現象。48 hpi 時,新萌發側根處有大量細菌聚集。在此位置,細菌最終會進入靠近側根原基穿透內皮層、皮層與表皮交界處的細胞。 側根的定殖相當稀疏,且僅限於側根裂縫處(圖 2d、e)。極少情況下,單個植物根部細胞會被細菌密集定殖(圖 2f)。在接種後 24 小時、48 小時和 6 天時,Oy-0、Ler-0 和 Col-0 的根部定殖模式未觀察到差異,這表明生長促進作用可能是由植物基因型對定殖的特異性反應所決定,而非細菌主動作用所致。
Confocal laser scanning micrographs of A. thaliana Oy-0 colonized by K. radicincitans expressing eGFP. (a) K. radicincitans colonizing a root hair. Image is a 13 μm stack, taken 24 hpi. (b) 14 μm stack image of K. radicincitans root surface colonization 48 hpi. (c) 13 μm stack of root surface 6 dpi. (d) Image showing a 36 μm stack taken 6 dpi of K. radicincitans colonizing the junctions of a lateral root primordium pushing through the epidermis. (e) K. radicincitans colonizing the junctions of a lateral root 6 dpi. (f) Very infrequently single cells are completely colonized by K. radicincitans. The image shows a 20 μm stack at 6 dpi. Scale bar: 20 μm
表達 eGFP 的 K. radicincitans 定殖於擬南芥 Oy-0 的共聚焦雷射掃描顯微照片。(a) K. radicincitans 定殖於根毛。影像為接種後 24 小時拍攝的 13 微米堆疊圖。(b) 接種後 48 小時 K. radicincitans 根部表面定殖的 14 微米堆疊圖。(c) 接種後 6 天根部表面的 13 微米堆疊圖。(d) 顯示接種後 6 天,K. radicincitans 定殖於側根原基突破表皮連接處的 36 微米堆疊圖。(e) 接種後 6 天 K. radicincitans 定殖於側根連接處。(f) 極罕見情況下,單個細胞會完全被 K. radicincitans 定殖。該影像為接種後 6 天拍攝的 20 微米堆疊圖。比例尺:20 微米
Presence of K. radicincitans affects root secondary metabolites
K. radicincitans 的存在影響根部次級代謝物
Secondary metabolites present in plant roots are known to govern plant-microbe interactions. Therefore, the composition and concentration of three major classes of secondary metabolites, glucosinolates, phenylpropanoids and carotenoids, were selected for profiling roots of Oy-0 as here the highest beneficial effects of bacterial inoculation were observed (see Fig. 1). Targeted analysis of glucosinolates allowed for the detection and quantification of 11 glucosinolates in roots of Oy-0, confirming earlier findings (Witzel et al. 2013). The amount of total glucosinolate content was lower in roots of inoculated plants and a statistically significant reduction of 8-(methylsulfinyl)octyl glucosinolate was found (Fig. 3a).
已知植物根部中的次級代謝物主導著植物與微生物的交互作用。因此,我們選擇對 Oy-0 品系的根部進行三類主要次級代謝物(硫代葡萄糖苷、苯丙烷類和類胡蘿蔔素)的組成與濃度分析,因為在此品系中觀察到最顯著的細菌接種效益(參見圖 1)。針對硫代葡萄糖苷的靶向分析,在 Oy-0 根部檢測並定量出 11 種硫代葡萄糖苷,此結果與先前研究一致(Witzel 等人 2013 年)。接種細菌的植株根部總硫代葡萄糖苷含量較低,其中 8-(甲硫基)辛基硫代葡萄糖苷的減少達到統計顯著性(圖 3a)。
The decline in glucosinolates (a) and phenylpropanoids (b) and the induction of carotenoids (c) by K. radicincitans colonization in A. thaliana roots. Values are given as mean ± standard error of three independent experiments, where each mean was derived from two technical replicates. Asterisks indicate statistical differences between inoculated and non-inoculated plants (p < 0.05)
K. radicincitans 定殖對阿拉伯芥根部造成硫代葡萄糖苷(a)與苯丙烷類(b)含量下降,以及類胡蘿蔔素(c)含量上升。數據以三次獨立實驗的平均值±標準誤表示,每次實驗均值來自兩次技術重複。星號標示接種與未接種植株間的統計顯著差異(p < 0.05)
The phenylpropanoids present in roots were identified and quantified using a HPLC coupled to an ion-trap mass spectrometer. Analysis allowed for the detection of seven metabolites present in the Oy-0 root. The coumarins, including scopoletin, scopoletin derivates (scopoletin-coniferylalcohol-glucoside, scopoletin-coniferylalcohol, a scopoletin derivative) and hydroxymethyl-coumarin, represented the largest quantitative group. In addition to that, cinnamic acids sinapoyl-glucoside and hydroxymethyl-coniferylalcohol-glucoside were detected. The levels of most compounds were lower in the inoculated plants and a statistically significant reduction was found for scopoletin-coniferylalcohol-glucoside (Fig. 3b).
根部中的苯丙素類化合物是使用高效液相層析串聯離子阱質譜儀進行鑑定與定量分析。該分析檢測出 Oy-0 根部含有七種代謝物。其中香豆素類(包括東莨菪素、東莨菪素衍生物[東莨菪素-松柏醇-葡萄糖苷、東莨菪素-松柏醇、一種東莨菪素衍生物]及羥甲基香豆素)在數量上佔最大比例。此外還檢測到肉桂酸衍生物芥子醯葡萄糖苷與羥甲基松柏醇葡萄糖苷。接種處理組中大多數化合物含量較低,其中東莨菪素-松柏醇-葡萄糖苷的減少具有統計顯著性(圖 3b)。
The lowest compound concentration was observed for carotenoids, which were analysed by UPLC-MS. The Oy-0 root contained α-lutein and β-carotene and, in response to the inoculation, both levels increased significantly (Fig. 3c).
類胡蘿蔔素的濃度最低,該物質是透過超高效液相層析質譜聯用技術進行分析。Oy-0 根部含有α-葉黃素與β-胡蘿蔔素,接種處理後兩者含量均顯著增加(圖 3c)。
K. radicincitans alters the rhizosecretion profile in Arabidopsis
放射形科薩克尼亞菌改變擬南芥的根部分泌物組成
The consequences of the widespread changes to plant secondary metabolites induced by the K. radicincitans colonization of roots were next explored with regard to compounds released into the rhizosphere by non-targeted analyses. GC-EI-Q-MS-based metabolite profiling revealed 32 compounds that were significantly affected (p < 0.05) by the presence of K. radicincitans (Table 1). Of these, 24 compounds were identified based on best mass spectral and retention index match (reverse match >500, retention index deviation <0.5%), and were shown to comprise several nucleobases, amino acids, organic acids, carbohydrates, polyols and phenylpropanoids. Except for lactic acid, all metabolites were reduced by inoculation. A parallel non-targeted LC-MS-based metabolite profiling approach revealed 1398 out of 3915 differentially affected (p < 0.05) unique mass-to-charge retention time pairs in positive ionization mode and 670 out of 2955 differential ones in negative ionization mode. The acquisition of collision-induced dissociation mass spectra of quasi-molecular ions, the application of on-column H/D chromatography and a comparison with mass spectral data from literature (Strehmel et al. 2014) successfully resulted in annotating 32 compounds (Table 2). The metabolites were linked to amino acids, dipeptides, aliphatic glucosinolate precursor amino acids, aliphatic glucosinolate degradation products, phenylpropanoids and diverse fatty acid derivatives, among others. The compounds associated to either glucosinolate or phenylpropanoid metabolisms were reduced in concentration by the inoculation, while fatty acid metabolites were enriched.
接著探討了 K. radicincitans 定殖根部所誘導的植物次級代謝物廣泛變化,對於釋放至根際化合物的影響,採用非靶向分析方法進行研究。基於 GC-EI-Q-MS 的代謝物分析顯示,有 32 種化合物受到 K. radicincitans 存在顯著影響(p < 0.05)(表 1)。其中 24 種化合物根據最佳質譜圖和保留指數匹配(反向匹配>500,保留指數偏差<0.5%)被鑑定出來,包含多種核鹼基、胺基酸、有機酸、碳水化合物、多元醇和苯丙素類化合物。除乳酸外,所有代謝物皆因接種而減少。平行進行的非靶向 LC-MS 代謝物分析顯示,在正離子模式下 3915 個獨特質荷比-保留時間對中有 1398 個存在差異影響(p < 0.05),負離子模式下 2955 個中有 670 個存在差異。通過獲取準分子離子的碰撞誘導解離質譜圖、應用柱上 H/D 色譜法,並與文獻質譜數據(Strehmel 等人)進行比對... 2014 年)成功註解了 32 種化合物(表 2)。這些代謝物與氨基酸、二肽、脂肪族硫代葡萄糖苷前體氨基酸、脂肪族硫代葡萄糖苷降解產物、苯丙素類化合物以及多種脂肪酸衍生物等相關。與硫代葡萄糖苷或苯丙素代謝途徑相關的化合物在接種後濃度降低,而脂肪酸代謝物則有所增加。
表 1 受 K. radicincitans 定殖影響的根系分泌物中差異積累初級植物代謝物
表 2 經 K. radicincitans 定殖後根部分泌物中差異積累的次級植物代謝物
In vitro effects of secondary metabolites on K. radicincitans growth
次級代謝物對 K. radicincitans 生長的體外影響
Several plant secondary metabolites showed an altered accumulation pattern in response to K. radicincitans inoculation. To test, whether this is a result of the plant’s adaptation to bacterial growth or it is a consequence of bacterial metabolic requirements, pure bacterial cultures were supplemented with pure representative compounds of glucosinolates (2-propenyl), carotenoids (lutein, β-carotene) and phenylpropanoids (scopoletin, sinapic acid). A fourth class of compounds was included to the assay and these were terpenes (squalene, α-humulene, farnesene) that also influence biotic interactions (Tholl 2015). Inhibitory effects on bacterial growth were observed for 2-propenyl, scopoletin, α-humulene and lutein (Fig. 4), while presence of sinapic acid, squalene and farnesene had no influence on K. radicincitans in vitro cell density. A beneficial effect was observed for β-carotene in the medium. Here, the highest supplied concentration of 100 μM resulted in an increased bacterial growth, which was 34% higher as compared to the control treatment, indicating that β-carotene might be metabolized by the bacterium or stimulate growth by other means.
多種植物次級代謝物在接種 K. radicincitans 後顯示出累積模式的改變。為測試此現象是植物對細菌生長的適應結果,抑或是細菌代謝需求所致,我們在純細菌培養中添加了硫代葡萄糖苷(2-丙烯基)、類胡蘿蔔素(葉黃素、β-胡蘿蔔素)和苯丙烷類(東莨菪亭、芥子酸)的代表性純化合物。實驗還納入第四類化合物——同樣影響生物間相互作用的萜烯類(角鯊烯、α-蓽澄茄油烯、法尼烯)(Tholl 2015)。結果顯示 2-丙烯基、東莨菪亭、α-蓽澄茄油烯和葉黃素對細菌生長具有抑制作用(圖 4),而芥子酸、角鯊烯和法尼烯的存在對 K. radicincitans 的體外細胞密度沒有影響。培養基中的β-胡蘿蔔素則表現出促進作用,當添加濃度達最高 100μM 時,細菌生長量較對照組增加 34%,表明β-胡蘿蔔素可能被該菌代謝或通過其他途徑刺激生長。
Response of K. radicincitans growth to plant secondary metabolites. Pure liquid cultures were supplemented with standard compounds of glucosinolate (a), carotenoids (b), phenylpropanoids (c) and terpenes (d). Bacterial growth was measured after 15 h and presented is the mean of two independent experiments ± standard error, related to the respective control. Capital letters denote significant difference among supplemented compounds and asterisks indicate statistical differences between control samples and supplemented samples (p < 0.05)
K. radicincitans 對植物次級代謝物的生長反應。純液體培養基中添加了以下標準化合物:硫代葡萄糖苷 (a)、類胡蘿蔔素 (b)、苯丙烷類化合物 (c) 和萜烯 (d)。細菌生長在 15 小時後測量,數據呈現為兩個獨立實驗的平均值±標準誤差,並與相應對照組進行比較。大寫字母表示添加化合物間的顯著差異,星號標示對照組與添加組樣本間的統計學差異 (p < 0.05)
Bacterial colonisation is dependent on presence of specific plant secondary metabolites
細菌定殖作用取決於特定植物次級代謝物的存在
To further define the role of secondary plant metabolites in the interaction with K. radicincitans, we tested the bacterial colonisation of plants disturbed in root secondary metabolite accumulation or synthesis: gtr1gtr2, sds1, ccoamt1, f6’h1. The double knockout of glucosinolate transporters 1 and 2, gtr1gtr2, accumulates one third of the wildtype amount of glucosinolates in roots and consequently releases lower amounts into the rhizosphere (Andersen et al. 2013; Xu et al. 2016). Solanesyl diphosphate synthase 1 (sds1) is involved in the synthesis of plastochinone (Liu and Lu 2016), an essential component of phytoene desaturation and therefore crucial for carotenoid biosynthesis (Chao et al. 2014). The sds1 knockout accumulates strongly reduced levels of carotenoids in leaves and roots (Supplemental Fig. S3). Caffeoyl-CoA-O-methyltransferase 1 (CCoAOMT1) is involved in lignin biosynthesis (Vanholme et al. 2012) and feruloyl-CoA-6-hydroxylase 1 (F6’H1) catalyses the synthesis of scopoletin which acts as iron chelator (Fourcroy et al. 2014) or phytoalexin (Sun et al. 2014). The root colonisation was assessed four days after inoculation by qPCR method and revealed considerable lower levels of K. radicincitans DNA on roots of sds1 and ccoaomt1 (Fig. 5). Levels of bacterial DNA were slightly higher on gtr1gtr2 and f6’h1 plants, but these changes were not statistically significant. No qPCR signals for K. radicincitans genomic DNA were obtained in samples from non-inoculated plants (not shown).
為進一步釐清次級植物代謝物在與放射根科薩克尼亞菌(K. radicincitans)交互作用中的角色,我們測試了根部次級代謝物累積或合成受阻之植物的細菌定殖情況:gtr1gtr2、sds1、ccoamt1、f6'h1。葡萄糖苷酸轉運蛋白 1 與 2 的雙重剔除株 gtr1gtr2,其根部僅累積野生型三分之一的硫代葡萄糖苷酸含量,因而釋放至根際的量也較少(Andersen 等人,2013;Xu 等人,2016)。茄尼基焦磷酸合成酶 1(sds1)參與質體醌合成(Liu 與 Lu,2016),此為八氫番茄紅素去飽和作用的必要成分,故對類胡蘿蔔素生合成至關重要(Chao 等人,2014)。sds1 剔除株在葉片與根部累積的類胡蘿蔔素水平顯著降低(補充圖 S3)。咖啡醯輔酶 A-O-甲基轉移酶 1(CCoAOMT1)參與木質素生合成(Vanholme 等人,2012),而阿魏醯輔酶 A-6-羥化酶 1(F6'H1)則催化作為鐵螯合劑(Fourcroy 等人,2014)或植物抗毒素(Sun 等人,2014)的東莨菪內酯合成。 接種後第四天透過定量聚合酶鏈式反應(qPCR)評估根部定殖情況,結果顯示 sds1 與 ccoaomt1 突變株根部所檢測到的 K. radicincitans DNA 含量顯著較低(圖 5)。gtr1gtr2 與 f6'h1 植株上的細菌 DNA 含量略高,但這些差異未達統計顯著性。未接種對照組植株樣本中未檢測到 K. radicincitans 基因組 DNA 的 qPCR 訊號(數據未顯示)。
K. radicincitans colonization of the roots of A. thaliana knock-out lines four days after inoculation, as detected by qPCR. Values represent the mean ± standard error (n = 3) of expression ratios (2–∆∆CT), normalized to two reference genes and to the inoculated Col-0. Capital letters denote significant differences between inoculated genotypes (p ≤ 0.05)
接種後第四天透過 qPCR 檢測 K. radicincitans 在阿拉伯芥基因敲除株系根部的定殖情況。數值表示經兩個參考基因標準化後,相對於接種 Col-0 對照組的表達比率(2 –∆∆CT )平均值±標準誤(n=3)。大寫字母標示不同接種基因型間具統計顯著差異(p≤0.05)
Discussion 討論
PGPB can contribute significantly to both crop productivity and plant health, but as yet modulation of the host’s physiology is poorly understood. Our results demonstrate that the plant genotype determines whether K. radicincitans induces a growth promotion in A. thaliana. This natural variation within a plant species in the response to PGPB inoculation was also reported earlier for 196 A. thaliana accession after P. fluorescens inoculation (Haney et al. 2015), a collection of 302 A. thaliana accessions tested with P. simiae (Wintermans et al. 2016) as well as for 40 Brachypodium distachyon accessions colonized by A. brasilense or H. seropedicae (do Amaral et al. 2016). The host genotypes Oy-0 and Ler-0 were most responsive, but diametrically opposed, to K. radicincitans inoculation, whereas the eGFP-based localisation assay revealed that roots of both accessions were well colonized by K. radicincitans. This effect has been found before (do Amaral et al. 2016) and demonstrates that growth promotion is a plant genotype-specific response to bacterial inoculation.
植物生長促進細菌(PGPB)對作物產量與植物健康具有重要貢獻,然而目前對於其調控宿主生理機制的理解仍相當有限。本研究結果顯示,擬南芥的基因型決定了 K. radicincitans 是否能誘導其生長促進效應。這種植物物種內部對 PGPB 接種反應的自然變異性,先前已在 196 個擬南芥品系對 P. fluorescens 接種(Haney 等人 2015)、302 個擬南芥品系對 P. simiae 測試(Wintermans 等人 2016),以及 40 個短柄草品系對 A. brasilense 或 H. seropedicae 定殖(do Amaral 等人 2016)的研究中被報導。宿主基因型 Oy-0 與 Ler-0 對 K. radicincitans 接種表現出最顯著但截然相反的反應,而基於 eGFP 的定位分析顯示兩個品系的根部均被 K. radicincitans 良好定殖。此現象先前已被發現(do Amaral 等人 2016),證實生長促進作用是植物基因型對細菌接種的特異性反應。
The host genotype governs the interaction with rhizobacteria through plant secondary metabolites present in the root and exuded to the rhizosphere (Drogue et al. 2012). Hence, we profiled major plant secondary metabolites in Oy-0 that may be positive regulators of this interaction (summarized in Fig. 6). We found a general decline in the concentration of root glucosinolate and phenylpropanoids in response to bacterial colonisation with significant differences for 8-(methylsulfinyl)octyl glucosinolate and scopoletin-coniferylalcohol-glucoside. Concomitant to this, levels of glucosinolate breakdown products and scopoletin-coniferylalcohol were lower in root exudates of inoculated plants. Glucosinolates are active in counteracting pathogen invasion (Brader et al. 2006), especially in their hydrolysed form (Kliebenstein 2004; Osbourn 1996). A regulatory role in structuring the rhizosphere microbial community was proposed for glucosinolates using transgenic A. thaliana (Bressan et al. 2009). The glucosinolate concentration of Brassica napus roots determined the colonisation density of the rhizobacterium Azorhizobium caulinodans (O'Callaghan et al. 2000). We showed that growth in the presence of different levels of 2-propenyl glucosinolate had no beneficial effects on cell density, while short-term bacterial root colonisation of gtr1gtr2 was slightly higher as compared to the wildtype, indicating that lower root glucosinolate levels might be favourable for bacterial colonisation. Comparative studies using A. thaliana transgenic lines over-accumulating or being devoid of glucosinolates should contribute to our knowledge on the role of this metabolite class in plant-bacteria interactions.
宿主基因型透過根部存在並分泌至根際的植物次級代謝物,調控與根際細菌的交互作用(Drogue 等人,2012)。因此,我們分析了 Oy-0 品系中可能正向調控此交互作用的主要植物次級代謝物(彙整於圖 6)。研究發現,在細菌定殖後,根部硫代葡萄糖苷與苯丙烷類化合物濃度普遍下降,其中 8-(甲基亞碸基)辛基硫代葡萄糖苷和東莨菪素-松柏醇-葡萄糖苷的變化達到顯著差異。與此同時,接種細菌的植物其根部分泌物中,硫代葡萄糖苷分解產物與東莨菪素-松柏醇的含量也較低。硫代葡萄糖苷具有抵禦病原體入侵的活性(Brader 等人,2006),特別是在其水解形態下(Kliebenstein,2004;Osbourn,1996)。透過轉基因擬南芥的研究,學者提出硫代葡萄糖苷在塑造根際微生物群落結構中可能具有調控作用(Bressan 等人,2009)。歐洲油菜(Brassica napus)根部的硫代葡萄糖苷濃度會影響根際細菌莖瘤固氮根菌(Azorhizobium caulinodans)的定殖密度(O'Callaghan 等人,2000)。 我們的研究顯示,在不同濃度的 2-丙烯基硫代葡萄糖苷環境中生長,對細胞密度並無正面影響,然而 gtr1gtr2 突變株的短期細菌根部定殖率略高於野生型,這表明較低的根部硫代葡萄糖苷含量可能有利於細菌定殖。透過比較研究過量累積或完全缺乏硫代葡萄糖苷的阿拉伯芥轉基因株系,應能增進我們對此類代謝物在植物-細菌交互作用中所扮演角色的理解。
Plant phenylpropanoids are intimately involved in pathogen- and oxidative stress defence. Scopoletin inhibits pathogen growth (Peterson et al. 2003), while scopolin, the glycosylated (inactive) form of scopoletin, promotes the growth of some fungi, and inhibits it in others (Ojala et al. 2000). Our study demonstrated the reduced presence of specific phenylpropanoids in root exudates and in the root itself. In order to test whether this might reflect the growth requirements of K. radicincitans, scopoletin and sinnapic acid were supplemented to the bacterial suspension. As for glucosinolates, their moderate inhibitory effects indicate that both substance classes cannot be metabolized by the bacterium. Hence, the decline upon plant inoculation is rather plant-driven than the result of bacterial metabolism and may be linked to the diverting of assimilates to primary metabolism. Short-term bacterial colonisation of ccoaomt1 was significantly lower as compared to Col-0 and f6’h1, indicating that presence of downstream products of ccoaomt1 are positive regulators for bacterial colonisation. However, no such correlation is established yet and it remains a hypothesis whether structural changes of cell wall or content of flavonol glycosides (Do et al. 2007) account for these observed effects.
植物苯丙烷類化合物與病原體防禦及氧化應激防禦密切相關。傘皮素能抑制病原體生長(Peterson 等人 2003 年研究),而其糖苷化形式(非活性態)的傘形酮苷則會促進某些真菌生長,同時抑制其他真菌(Ojala 等人 2000 年研究)。本研究證實特定苯丙烷類化合物在根系分泌物及根部組織中的含量降低。為驗證此現象是否反映 K. radicincitans 菌株的生長需求,我們在細菌懸浮液中添加了傘形酮及芥子酸。就硫代葡萄糖苷而言,其適度抑制作用顯示這兩類物質均無法被該菌株代謝利用。因此,接種後含量下降應歸因於植物自身調控而非細菌代謝作用,可能與同化產物轉向初級代謝有關。ccoaomt1 突變體的短期細菌定殖量顯著低於 Col-0 與 f6'h1,表明 ccoaomt1 下游產物是促進細菌定殖的正向調節因子。 然而,目前尚未建立此類相關性,關於細胞壁結構變化或黃酮醇苷含量(Do 等人,2007 年)是否導致這些觀察到的效應,仍僅止於假設階段。
Carotenoids are associated with light-harvesting, photoprotection, photosensing and antioxidant protection in the leaf, while their degradation products control biotic interactions in roots (Walter et al. 2010). Reduced apocarotenoid levels in transgenic tomato had negative effects on root colonisation with arbuscular mycorrhizal fungi (AMF) (Kohlen et al. 2012) and C13 apocarotenoids act to support the functionality of the AMF symbiosis during later stages of interaction (Walter 2013). The inoculation of A. thaliana with K. radicincitans raised the carotenoid content of the root, and in vitro testing revealed that β-carotene increases bacterial cell density. Whether β-carotene is metabolized and it’s cleavage products act as signalling compounds or if the antioxidant properties play a role in the plant-bacterium interaction, remains to be determined. Our findings, that bacterial colonisation of sds1 was reduced as compared to the wildtype Col-0, strengthens the assumption that carotenoid-derived compounds play an important role in establishing K. radicincitans colonisation in A. thaliana.
類胡蘿蔔素在葉片中與光能捕獲、光保護、光感應及抗氧化保護作用相關,而其降解產物則調控根部生物互作(Walter 等人,2010)。轉基因番茄中降低的類胡蘿蔔素裂解產物水平對叢枝菌根真菌(AMF)的根部定殖產生負面影響(Kohlen 等人,2012),且 C 13 類胡蘿蔔素裂解產物在互作後期階段支持 AMF 共生功能(Walter,2013)。接種 K. radicincitans 使阿拉伯芥根部類胡蘿蔔素含量上升,體外試驗顯示β-胡蘿蔔素能增加細菌細胞密度。至於β-胡蘿蔔素是否被代謝、其裂解產物是否作為信號分子發揮作用,或是其抗氧化特性在植物-細菌互作中扮演角色,仍有待釐清。我們發現 sds1 突變體的細菌定殖量較野生型 Col-0 減少,此結果強化了類胡蘿蔔素衍生物在建立 K. radicincitans 於阿拉伯芥定殖過程中具有重要作用的假設。
The root exudates of inoculated plants contained less carbohydrate, amino acid, organic acid and nucleobase, as was also the case in both tobacco and groundnut colonized by Bacillus cereus (Dutta et al. 2013), and in tomato colonized by P. fluorescens (Kamilova et al. 2006). When supplied with tomato root exudates, P. fluorescence WCS365 reduces the amounts of sugars, especially ribose and glucose, and of organic acids, such as citric acid, malic acid and fumaric acid (Kamilova et al. 2006). The PGPB Enterobacter sp. strain 638 requires the host to supply glucose, cellobiose or xylose as a source of carbon (Taghavi et al. 2010), and in vitro cultured K. radicincitans metabolizes these same carbohydrates (Kämpfer et al. 2005). Therefore, a reduction in the exudate of these metabolites in PGPB colonized roots could reflect their metabolisation by the bacteria. Another explanation for altered root exudation pattern could be a PGPB-mediated reduction in exudation, as shown for potato inoculated with rhizobacteria (Belimov et al. 2015).
接種植物的根系分泌物含有較少的碳水化合物、胺基酸、有機酸和核鹼基,這種情況也出現在被蠟樣芽孢桿菌(Bacillus cereus)定殖的菸草和花生(Dutta 等人 2013 年),以及被螢光假單胞菌(P. fluorescens)定殖的番茄中(Kamilova 等人 2006 年)。當提供番茄根系分泌物時,螢光假單胞菌 WCS365 會減少糖類(尤其是核糖和葡萄糖)以及有機酸(如檸檬酸、蘋果酸和富馬酸)的含量(Kamilova 等人 2006 年)。植物生長促進細菌 Enterobacter sp.菌株 638 需要宿主提供葡萄糖、纖維二糖或木糖作為碳源(Taghavi 等人 2010 年),而體外培養的 K. radicincitans 也會代謝這些相同的碳水化合物(Kämpfer 等人 2005 年)。因此,在 PGPB 定殖的根系中這些代謝物分泌量的減少,可能反映了細菌對它們的代謝作用。另一種解釋根系分泌物模式改變的原因可能是 PGPB 介導的分泌減少,如接種根際細菌的馬鈴薯所示(Belimov 等人 2015 年)。
Only a limited number of metabolites was enriched in the root exudate of the inoculated plants, e.g. lactic acid. In soybean roots, infection with Bradyrhizobium japonicum has been shown to promote the lactic acid content of the root hair (Brechenmacher et al. 2010), while lactic acid is also accumulated in the Sinorhizobium meliloti nodules attached to the alfalfa root (Barsch et al. 2006). The function of lactic acid accumulation during symbiotic interactions is vague, and in particular, it is unclear whether the lactic acid is produced by the host and/or by the bacterium. Four derivatives of the fatty acids were prominent in the inoculated A. thaliana root exudate: the hydroxylated and oxidized form of the dicarboxylic undecanoic acid, the monounsaturated oxodecenoic acid and the unsaturated long-chain tetradecadienedioic acid. Fatty acids are involved in cell membrane composition, membrane trafficking and signal transduction in the plant cell. However, they may also have a role in extracellular communication with microorganisms, as shown for example by their ability to disrupt the biofilms formed by soil bacteria and fungi (Davies and Marques 2009). Tetradecanoic acid is synthesized by both P. putida and P. aeruginosa, and is also present in root exudates of maize (Fernandez-Pinar et al. 2012). This fatty acid is known to activate the expression of ddcA, a gene required for the colonization of both the seed and root by P. putida (Espinosa-Urgel and Ramos 2004). The Brechenmacher et al. (2010) study of the soybean metabolome also demonstrated the increased abundance of six different fatty acids in the root hair, but not in stripped roots, during the early phase of the root’s interaction with B. japonicum. The data suggest that specific fatty acid derivatives might be involved in the rhizosphere communication, which could also hold true for K. radicincitans.
接種植物的根系分泌物中僅有少量代謝物含量增加,例如乳酸。在大豆根部研究中,接種慢生根瘤菌(Bradyrhizobium japonicum)已被證實會促進根毛中乳酸含量(Brechenmacher 等,2010),而附著於苜蓿根部的苜蓿中華根瘤菌(Sinorhizobium meliloti)根瘤中也會累積乳酸(Barsch 等,2006)。乳酸在共生交互作用期間的累積功能尚不明確,特別是無法確定乳酸是由宿主或細菌所產生。在接種阿拉伯芥(A. thaliana)的根系分泌物中,有四種脂肪酸衍生物表現顯著:雙羧基十一烷酸的羥基化與氧化形式、單不飽和氧代癸烯酸以及不飽和長鏈十四碳二烯二酸。脂肪酸參與植物細胞的細胞膜組成、膜運輸與信號傳導,但可能也涉及與微生物的胞外通訊,例如其破壞土壤細菌與真菌形成生物膜的能力(Davies 與 Marques,2009)。 十四烷酸(Tetradecanoic acid)可由綠膿桿菌(P. putida)與銅綠假單胞菌(P. aeruginosa)合成,同時也存在於玉米根系分泌物中(Fernandez-Pinar 等學者 2012 年研究)。此脂肪酸已知能激活 ddcA 基因表現,該基因對綠膿桿菌於種子與根部的定殖過程不可或缺(Espinosa-Urgel 與 Ramos 2004 年研究)。Brechenmacher 等學者(2010 年)針對大豆代謝體的研究亦證實,在根部與大豆根瘤菌(B. japonicum)相互作用的初期階段,根毛組織(非剝離根部)中六種不同脂肪酸的豐度會增加。這些數據顯示特定脂肪酸衍生物可能參與根際通訊機制,此現象或許同樣適用於放射性柯薩克氏菌(K. radicincitans)。
In conclusion, the exploitation of beneficial microbes in the context of devising a more sustainable regime of crop fertilization will require an in-depth understanding of how PGPBs interact with the host. We have reported here how colonization of A. thaliana by K. radicincitans altered the root exudation pattern and the profile of secondary metabolites accumulated in a host with a high level of growth promotion. Some of these metabolites are known to function as a carbon source for bacteria, while for others, no role in the plant-bacteria interaction has as yet been established. Furthermore, an increasing number of studies demonstrate the genotype-specific diversity of the host-associated microbiome and its impact on plant health (Haney et al. 2015; Schlaeppi et al. 2014; Zachow et al. 2014). Hence, it can be assumed that also microbes present in the endosphere or rhizosphere affect the plant-growth-promoting ability of K. radicincitans. Wheat inoculated with K. radicincitans was stronger colonized when plants were grown in sterilized soil as compared to non-sterilized soil and four tested wheat cultivars revealed different intensity levels of colonisation (Remus et al. 2000). Studies on the genotype-specific constitution of the plant microbiome and its influence on PGPB efficiency will contribute to our understanding of the complex plant-microbiome interaction.
總而言之,在設計更永續的作物施肥方案時,若要有效利用有益微生物,必須深入理解植物生長促進細菌(PGPB)與宿主間的互動機制。本研究揭示了 K. radicincitans 定殖於阿拉伯芥後,如何改變根系分泌模式及具有高度生長促進效應宿主體內次級代謝物的累積特徵。其中部分代謝物已知可作為細菌的碳源,而其他代謝物在植物-細菌互作中的功能尚未明確。此外,越來越多研究證實宿主相關微生物組具有基因型特異性多樣性,並影響植物健康(Haney 等,2015;Schlaeppi 等,2014;Zachow 等,2014)。據此可推測,內生圈或根際中的微生物亦會影響 K. radicincitans 的植物生長促進能力。與未滅菌土壤相比,接種 K. radicincitans 的小麥在滅菌土壤中表現出更強的定殖現象,且四種測試小麥品種顯示出不同強度的定殖程度(Remus 等,2000)。 關於植物微生物組基因型特異性組成及其對植物生長促進細菌(PGPB)效率影響的研究,將有助於我們理解複雜的植物-微生物組相互作用。
Abbreviations 縮寫詞
- AMF: AMF:
-
Arbuscular mycorrhizal fungi
叢枝菌根真菌 - CLSM: 共軛焦雷射掃描顯微鏡
-
Confocal laser scanning microscopy
共軛焦雷射掃描顯微鏡 - eGFP: 增強型綠色螢光蛋白
-
Enhanced green fluorescent proteinPGBP plant growth promoting bacteria
增強型綠色螢光蛋白 PGBP 植物生長促進細菌 - GC-EI-Q-MS: 氣相層析-電子游離-四極桿質譜儀
-
Gas chromatography-electron ionization-quadrupole mass spectrometry
氣相層析-電子游離-四極桿質譜法 - HPLC-DAD-ESI-MSn : 高效液相層析-二極體陣列偵測-電噴灑游離-多級質譜儀
-
High-performance liquid chromatography-diode array detection-electrospray ionization-multiple stage mass spectrometry
高效液相層析-二極體陣列偵測-電噴灑游離-多級質譜法 - IAA: IAA(吲哚-3-乙酸)
-
Indole-3-acetic acid 吲哚-3-乙酸
- UPLC-ESI-QTOF-MS: 超高效液相層析-電噴灑游離-四極桿飛行時間質譜儀
-
Ultra-performance liquid chromatography-electrospray ionization-quadrupole time of flight-mass spectrometry
超高效能液相層析-電噴灑游離-四極桿飛行時間質譜儀
References 參考文獻
Andersen TG, Nour-Eldin HH, Fuller VL, Olsen CE, Burow M, Halkier BA (2013) Integration of biosynthesis and long-distance transport establish organ-specific glucosinolate profiles in vegetative Arabidopsis. Plant Cell 25:3133–3145
Andersen TG、Nour-Eldin HH、Fuller VL、Olsen CE、Burow M、Halkier BA (2013) 生物合成與長距離運輸的整合建立了擬南芥營養器官特異性芥子油苷分布模式。植物細胞 25:3133–3145Baldermann S, Kato M, Kurosawa M, Kurobayashi Y, Fujita A, Fleischmann P, Watanabe N (2010) Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J Exp Bot 61:2967–2977
Baldermann S, Kato M, Kurosawa M, Kurobayashi Y, Fujita A, Fleischmann P, Watanabe N (2010) 木犀花發育過程中類胡蘿蔔素裂解雙加氧酶 1 的功能特性及其與類胡蘿蔔素累積和揮發性物質釋放的關係。實驗植物學雜誌 61:2967–2977Barsch A, Tellström V, Patschkowski T, Küster H, Niehaus K (2006) Metabolite profiles of nodulated alfalfa plants indicate that distinct stages of nodule organogenesis are accompanied by global physiological adaptations. Mol Plant-Microbe Interact 19:998–1013
Barsch A、Tellström V、Patschkowski T、Küster H、Niehaus K (2006) 結瘤苜蓿植株的代謝物譜分析顯示,根瘤器官發生的不同階段伴隨著整體生理適應。分子植物-微生物相互作用 19:998–1013Belimov AA, Dodd IC, Safronova VI, Shaposhnikov AI, Azarova TS, Makarova NM, Davies WJ, Tikhonovich IA (2015) Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann Appl Biol 167:11–25
Belimov AA、Dodd IC、Safronova VI、Shaposhnikov AI、Azarova TS、Makarova NM、Davies WJ、Tikhonovich IA (2015) 產生生長素並含有 1-氨基環丙烷-1-羧酸脫氨酶的根際細菌降低根際氨基酸濃度並促進充分灌溉與水分限制條件下馬鈴薯(Solanum tuberosum)的生長與產量。應用生物學年報 167:11–25Berg G (2009) Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18
伯格 G (2009) 促進植物生長與健康的植物-微生物交互作用:農業中微生物控制應用的前景。《應用微生物學與生物技術》84:11–18Berger B, Wiesner M, Brock AK, Schreiner M, Ruppel S (2015) K. radicincitans, a beneficial bacteria that promotes radish growth under field conditions. Agron Sustain Dev 35:1521–1528
Berger B, Wiesner M, Brock AK, Schreiner M, Ruppel S (2015) 促進蘿蔔生長的益菌 K. radicincitans 於田間條件下的研究。Agron Sustain Dev 35:1521–1528Berger B, Baldermann S, Ruppel S (2017) The plant growth-promoting bacterium Kosakonia radicincitans improves fruit yield and quality of Solanum lycopersicum. J Sci Food Agric: Epub ahead of print, doi:10.1002/jsfa.8357
Berger B、Baldermann S、Ruppel S(2017)促進植物生長之細菌 Kosakonia radicincitans 提升番茄(Solanum lycopersicum)果實產量與品質。《農業與食品科學期刊》:線上預先出版,doi: 10.1002/jsfa.8357Bertin C, Yang XH, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Bertin C、Yang XH、Weston LA(2003)根系分泌物與化感物質在根際圈中的作用。《植物與土壤》256:67–83Bloemberg GV, Wijfjes AHM, Lamers GEM, Stuurman N, Lugtenberg BJJ (2000) Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: new perspectives for studying microbial communities. Mol Plant-Microbe Interact 13:1170–1176
Bloemberg GV、Wijfjes AHM、Lamers GEM、Stuurman N、Lugtenberg BJJ (2000) 同時顯影表現三種不同自體螢光蛋白的螢光假單胞菌 WCS365 族群在根際的分佈:研究微生物群落的新視角。Mol Plant-Microbe Interact 13:1170–1176Bodenhausen N, Horton MW, Bergelson J (2013) Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS One 8:e56329
Bodenhausen N, Horton MW, Bergelson J (2013) 阿拉伯芥葉片與根部相關之細菌群落。PLoS One 8:e56329Brader G, Mikkelsen MD, Halkier BA, Palva ET (2006) Altering glucosinolate profiles modulates disease resistance in plants. Plant J 46:758–767
Brader G, Mikkelsen MD, Halkier BA, Palva ET (2006) 改變硫代葡萄糖苷譜可調節植物抗病性。Plant J 46:758–767Brady C, Cleenwerck I, Venter S, Coutinho T, De Vos P (2013) Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst Appl Microbiol 36:309–319
Brady C、Cleenwerck I、Venter S、Coutinho T、De Vos P (2013) 基於多位點序列分析(MLSA)對腸桿菌屬進行分類學評估:建議將 E. nimipressuralis 和 E. amnigenus 重新分類為新屬 Lelliottia,分別命名為 Lelliottia nimipressuralis comb. nov.和 Lelliottia amnigena comb. nov.;將 E. gergoviae 和 E. pyrinus 歸入新屬 Pluralibacter,分別命名為 Pluralibacter gergoviae comb. nov.和 Pluralibacter pyrinus comb. nov.;將 E. cowanii、E. radicincitans、E. oryzae 和 E. arachidis 歸入新屬 Kosakonia,分別命名為 Kosakonia cowanii comb. nov.、Kosakonia radicincitans comb. nov.、Kosakonia oryzae comb. nov.和 Kosakonia arachidis comb. nov.;以及將 E. turicensis、E. helveticus 和 E. pulveris 歸入 Cronobacter 屬,分別命名為 Cronobacter zurichensis nom. nov.、Cronobacter helveticus comb. nov.和 Cronobacter pulveris comb. nov.,並修訂腸桿菌屬與 Cronobacter 屬的描述。系統與應用微生物學 36:309–319Brechenmacher L, Lei Z, Libault M, Findley S, Sugawara M, Sadowsky MJ, Sumner LW, Stacey G (2010) Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. Plant Physiol 153:1808–1822
Brechenmacher L、Lei Z、Libault M、Findley S、Sugawara M、Sadowsky MJ、Sumner LW、Stacey G (2010) 大豆根毛代謝物受共生菌 Bradyrhizobium japonicum 調節之研究。植物生理學 153:1808–1822Bressan M, Roncato MA, Bellvert F, Comte G, Haichar FE, Achouak W, Berge O (2009) Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. Isme J 3:1243–1257
Bressan M、Roncato MA、Bellvert F、Comte G、Haichar FE、Achouak W、Berge O (2009) 阿拉伯芥產生的外源硫代葡萄糖苷對根際微生物和植物根系的影響。《國際微生物生態學會期刊》3:1243–1257Brock A, Berger B, Mewis I, Ruppel S (2013) Impact of the PGPB Enterobacter radicincitans DSM 16656 on growth, glucosinolate profile, and immune responses of Arabidopsis thaliana. Microb Ecol 65:661–670
Brock A, Berger B, Mewis I, Ruppel S (2013) 植物生長促進菌 Enterobacter radicincitans DSM 16656 對擬南芥生長、硫代葡萄糖苷譜及免疫反應的影響。微生物生態學 65:661–670Cartieaux F, Thibaud MC, Zimmerli L, Lessard P, Sarrobert C, David P, Gerbaud A, Robaglia C, Somerville S, Nussaume L (2003) Transcriptome analysis of Arabidopsis colonized by a plant-growth promoting rhizobacterium reveals a general effect on disease resistance. Plant J 36:177–188
Cartieaux F、Thibaud MC、Zimmerli L、Lessard P、Sarrobert C、David P、Gerbaud A、Robaglia C、Somerville S、Nussaume L (2003) 促進植物生長根際菌定殖阿拉伯芥之轉錄組分析揭示其對病害抗性之普遍效應。《植物學期刊》36:177–188Chamam A, Sanguin H, Bellvert F, Meiffren G, Comte G, Wisniewski-Dyé F, Bertrand C, Prigent-Combaret C (2013) Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum–Oryza sativa association. Phytochemistry 87:65–77
Chamam A, Sanguin H, Bellvert F, Meiffren G, Comte G, Wisniewski-Dyé F, Bertrand C, Prigent-Combaret C (2013) 植物次級代謝物分析證實固氮螺菌-水稻共生關係中的菌株依賴效應。植物化學 87:65–77Chao Y, Kang J, Zhang T, Yang Q, Gruber MY, Sun Y (2014) Disruption of the homogentisate solanesyltransferase gene results in albino and dwarf phenotypes and root, trichome and stomata defects in Arabidopsis Thaliana. PLoS One 9:e94031
Chao Y, Kang J, Zhang T, Yang Q, Gruber MY, Sun Y (2014) 破壞高龍膽酸茄尼基轉移酶基因導致阿拉伯芥出現白化與矮化表型及根毛、表皮毛與氣孔缺陷。PLoS One 9:e94031Compant S, Clement C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Compant S, Clement C, Sessitsch A (2010) 植物根際與內生圈中的植物生長促進細菌:其作用、定殖機制及應用前景。《土壤生物學與生物化學》42:669–678Davies DG, Marques CNH (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403
Davies DG, Marques CNH (2009) 一種脂肪酸訊息分子負責誘導微生物生物膜的分散。細菌學雜誌 191:1393–1403do Amaral FP, VCS P, ACM A, de Souza EM, Pedrosa F, Stacey G (2016) Differential growth responses of Brachypodium distachyon genotypes to inoculation with plant growth promoting rhizobacteria. Plant Mol Biol 90:689–697
do Amaral FP、VCS P、ACM A、de Souza EM、Pedrosa F、Stacey G (2016) 不同基因型短柄草對植物根際促生菌接種的差異生長反應。植物分子生物學 90:689–697Do C-T, Pollet B, Thévenin J, Sibout R, Denoue D, Barrière Y, Lapierre C, Jouanin L (2007) Both caffeoyl coenzyme a 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 226:1117–1129
Do C-T、Pollet B、Thévenin J、Sibout R、Denoue D、Barrière Y、Lapierre C、Jouanin L (2007) 咖啡醯輔酶 A 3-O-甲基轉移酶 1 與咖啡酸 O-甲基轉移酶 1 在阿拉伯芥中對木質素、類黃酮及芥子醯蘋果酸生合成具有重疊功能。Planta 226:1117–1129Dodd IC, Zinovkina NY, Safronova VI, Belimov AA (2010) Rhizobacterial mediation of plant hormone status. Ann Appl Biol 157:361–379
Dodd IC, Zinovkina NY, Safronova VI, Belimov AA (2010) 根際細菌對植物激素狀態的調控作用。應用生物學年報 157:361–379Drogue B, Doré H, Borland S, Wisniewski-Dyé F, Prigent-Combaret C (2012) Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res Microbiol 163:500–510
Drogue B, Doré H, Borland S, Wisniewski-Dyé F, Prigent-Combaret C (2012) 植物促生根際細菌與植物間合作的特異性為何? Res Microbiol 163:500–510Dutta S, Rani TS, Podile AR (2013) Root exudate-induced alterations in Bacillus cereus cell wall contribute to root colonization and plant growth promotion. PLoS One 8:e78369
Dutta S, Rani TS, Podile AR (2013) 根系分泌物誘導的蠟樣芽孢桿菌細胞壁改變有助於根部定殖與植物生長促進。PLoS One 8:e78369El Zemrany H, Czarnes S, Hallett PD, Alamercery S, Bally R, Jocteur Monrozier L (2007) Early changes in root characteristics of maize (Zea Mays) following seed inoculation with the PGPR Azospirillum lipoferum CRT1. Plant Soil 291:109–118
El Zemrany H、Czarnes S、Hallett PD、Alamercery S、Bally R、Jocteur Monrozier L (2007) 玉米(Zea Mays)種子接種 PGPR Azospirillum lipoferum CRT1 後根部特性的早期變化。植物與土壤 291:109–118Espinosa-Urgel M, Ramos JL (2004) Cell density-dependent gene contributes to efficient seed colonization by Pseudomonas putida KT2440. Appl Environ Microbiol 70:5190–5198
Espinosa-Urgel M, Ramos JL (2004) 細胞密度依賴性基因促進惡臭假單胞菌 KT2440 對種子的高效定殖。應用與環境微生物學 70:5190–5198Faure D, Vereecke D, Leveau JHJ (2009) Molecular communication in the rhizosphere. Plant Soil 321:279–303
Faure D, Vereecke D, Leveau JHJ (2009) 根際中的分子通訊。Plant Soil 321:279–303Fernandez-Pinar R, Espinosa-Urgel M, Dubern JF, Heeb S, Ramos JL, Camara M (2012) Fatty acid-mediated signalling between two Pseudomonas species. Environ Microbiol Rep 4:417–423
Fernandez-Pinar R, Espinosa-Urgel M, Dubern JF, Heeb S, Ramos JL, Camara M (2012) 兩種假單胞菌屬物種間的脂肪酸介導信號傳導。環境微生物學報告 4:417–423Fourcroy P, Siso-Terraza P, Sudre D, Saviron M, Reyt G, Gaymard F, Abadia A, Abadia J, Alvarez-Fernandez A, Briat JF (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201:155–167
Fourcroy P, Siso-Terraza P, Sudre D, Saviron M, Reyt G, Gaymard F, Abadia A, Abadia J, Alvarez-Fernandez A, Briat JF (2014) 擬南芥根部 ABCG37 轉運蛋白參與缺鐵條件下東莨菪素及其衍生物分泌之研究。新植物學家 201:155–167Gibeaut DM, Hulett J, Cramer GR, Seemann JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115:317–319
Gibeaut DM、Hulett J、Cramer GR、Seemann JR (1997) 使用簡易低維護水耕法與適宜環境條件達成阿拉伯芥最大生物量。植物生理學 115:317–319Haney CH, Samuel BS, Bush J, Ausubel FM (2015) Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat Plants 1:1–9
Haney CH、Samuel BS、Bush J、Ausubel FM(2015)與根際細菌的共生關係可賦予植物適應優勢。《自然植物》1:1–9Höflich G, Ruppel S (1994) Growth stimulation of pea after inoculation with associative bacteria. Microbiol Res 149:99–104
Höflich G、Ruppel S(1994)接種共生細菌對豌豆生長的促進作用。《微生物研究》149:99–104Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg B (2006) Effects of the tomato pathogen Fusarium oxysporum f. Sp. radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol Plant-Microbe Interact 19:1121–1126
Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg B (2006) 番茄病原菌 Fusarium oxysporum f. Sp. radicis-lycopersici 與生物防治菌 Pseudomonas fluorescens WCS365 對番茄根系分泌物中有機酸與糖類組成的影響。Mol Plant-Microbe Interact 19:1121–1126Kämpfer P, Ruppel S, Remus R (2005) Enterobacter radicincitans sp nov., a plant growth promoting species of the family Enterobacteriaceae. Syst Appl Microbiol 28:213–221
Kämpfer P、Ruppel S、Remus R (2005) Enterobacter radicincitans sp nov.,一種屬於腸桿菌科的植物生長促進菌種。系統與應用微生物學 28:213–221Kliebenstein DJ (2004) Secondary metabolites and plant/environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ 27:675–684
Kliebenstein DJ (2004) 次級代謝產物與植物/環境交互作用:透過阿拉伯芥視角的觀察。Plant Cell Environ 27:675–684Kohlen W, Charnikhova T, Lammers M, Pollina T, Tóth P, Haider I, Pozo MJ, de Maagd RA, Ruyter-Spira C, Bouwmeester HJ, López-Ráez JA (2012) The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 196:535–547
Kohlen W, Charnikhova T, Lammers M, Pollina T, Tóth P, Haider I, Pozo MJ, de Maagd RA, Ruyter-Spira C, Bouwmeester HJ, López-Ráez JA (2012) 番茄類胡蘿蔔素裂解雙加氧酶 8(SlCCD8)通過獨腳金內酯生物合成調控根際信號傳導、植株構型並影響生殖發育。《新植物學家》196:535–547Lagendijk EL, Validov S, Lamers GEM, de Weert S, Bloemberg GV (2010) Genetic tools for tagging gram-negative bacteria with mCherry for visualization in vitro and in natural habitats, biofilm and pathogenicity studies. FEMS Microbiol Lett 305:81–90
Lagendijk EL、Validov S、Lamers GEM、de Weert S、Bloemberg GV (2010) 以 mCherry 標記革蘭氏陰性菌的遺傳工具用於體外與自然棲地中的可視化、生物膜及致病性研究。《FEMS 微生物學快報》305:81–90Lakshmanan V, Castaneda R, Rudrappa T, Bais HP (2013) Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 238:657–668
Lakshmanan V、Castaneda R、Rudrappa T、Bais HP (2013) 阿拉伯芥暴露於有益枯草芽孢桿菌 FB17 根際細菌後的根部轉錄組分析揭示了與蘋果酸外排無關的細菌招募與植物防禦基因。《Planta》238:657–668Liu M, Lu S (2016) Plastoquinone and ubiquinone in plants: biosynthesis, physiological function and metabolic engineering. Front Plant Sci 7:1898
劉明、盧松 (2016) 植物中的質體醌與泛醌:生物合成、生理功能與代謝工程。植物科學前沿 7:1898Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Livak KJ, Schmittgen TD (2001) 使用即時定量 PCR 和 2(−Delta Delta C( T ))方法分析相對基因表現數據。方法 25:402–408Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol, Annual Reviews, Palo Alto
Lugtenberg B, Kamilova F (2009) 促進植物生長的根際細菌。Annu Rev Microbiol, Annual Reviews, Palo AltoMatsuoka H, Akiyama M, Kobayashi K, Yamaji K (2013) Fe and P solubilization under limiting conditions by bacteria isolated from Carex kobomugi roots at the Hasaki coast. Curr Microbiol 66:314–321
松岡博、秋山真由美、小林健一、山路和樹 (2013) 在限制條件下由濱刺草根部分離之細菌對鐵與磷的溶解作用。當代微生物學 66:314–321Narula N, Kothe E, Behl RK (2009) Role of root exudates in plant-microbe interactions. J Appl Bot Food Qual-Angew Bot 82:122–130
Narula N, Kothe E, Behl RK (2009) 根系分泌物在植物-微生物相互作用中的角色。應用植物學與食品品質雜誌-德國植物學 82:122–130Neugart S, Kläring HP, Zietz M, Schreiner M, Rohn S, Kroh LW, Krumbein A (2012) The effect of temperature and radiation on flavonol aglycones and flavonol glycosides of kale (Brassica oleracea Var. sabellica). Food Chem 133:1456–1465
Neugart S、Kläring HP、Zietz M、Schreiner M、Rohn S、Kroh LW、Krumbein A (2012) 溫度和輻射對羽衣甘藍(Brassica oleracea Var. sabellica)中黃酮醇苷元及黃酮醇苷的影響。食品化學 133:1456–1465O'Callaghan KJ, Stone PJ, Hu XJ, Griffiths DW, Davey MR, Cocking EC (2000) Effects of glucosinolates and flavonoids on colonization of the roots of Brassica napus by Azorhizobium caulinodans ORS571. Appl Environ Microbiol 66:2185–2191
O'Callaghan KJ、Stone PJ、Hu XJ、Griffiths DW、Davey MR、Cocking EC (2000) 葡萄糖苷酸鹽與類黃酮對 Azorhizobium caulinodans ORS571 於油菜(Brassica napus)根部定殖之影響。應用與環境微生物學 66:2185–2191Ojala T, Remes S, Haansuu P, Vuorela H, Hiltunen R, Haahtela K, Vuorela P (2000) Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J Ethnopharmacol 73:299–305
Ojala T, Remes S, Haansuu P, Vuorela H, Hiltunen R, Haahtela K, Vuorela P (2000) 芬蘭生長含香豆素藥用植物的抗菌活性研究。民族藥理學雜誌 73:299–305Osbourn AE (1996) Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8:1821–1831
Osbourn AE (1996) 植物預先形成的抗微生物化合物與抵禦真菌侵襲之機制。植物細胞 8:1821–1831Peterson JK, Harrison HF, Jackson DM, Snook ME (2003) Biological activities and contents of scopolin and scopoletin in sweetpotato clones. Hortscience 38:1129–1133
彼得森 JK、哈里森 HF、傑克森 DM、斯努克 ME(2003)甘藷品系中東莨菪苷與東莨菪素的生物活性及含量分析。《園藝科學》38 卷:1129-1133 頁Poupin MJ, Timmermann T, Vega A, Zuniga A, Gonzalez B (2013) Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS One 8:e69435
普潘 MJ、提默曼 T、維加 A、祖尼加 A、岡薩雷斯 B(2013)植物促生菌 Burkholderia phytofirmans PsJN 對擬南芥全生命週期之影響。《公共科學圖書館·綜合》8 卷:e69435Remus R, Ruppel S, Jacob HJ, Hecht-Buchholz C, Merbach W (2000) Colonization behaviour of two enterobacterial strains on cereals. Biol Fertility Soils 30:550–557
Remus R, Ruppel S, Jacob HJ, Hecht-Buchholz C, Merbach W (2000) 兩種腸道細菌在穀類作物上的定殖行為。生物肥沃土壤 30:550–557Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability. Plant Physiol 156:989–996
Richardson AE, Simpson RJ (2011) 調控磷有效性的土壤微生物。植物生理學 156:989–996Rosenblueth M, Martinez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant-Microbe Interact 19:827–837
Rosenblueth M, Martinez-Romero E (2006) 細菌內生菌及其與宿主的相互作用。Mol Plant-Microbe Interact 19:827–837Ruppel S (1988) Isolation and characterization of dinitrogen-fixing bacteria from the rhizosphere of Triticum aestivum and Ammophila arenaria. In: Vancura V, Kunc F (eds) Interrelationships between microorganisms and plants in soil: developments in soil science. Elsevier Science Publishers, Amsterdam
Ruppel S (1988) 從小麥(Triticum aestivum)和濱草(Ammophila arenaria)根際分離並鑑定固氮細菌。收錄於:Vancura V, Kunc F (編) 《土壤中微生物與植物間的相互關係:土壤科學進展》。Elsevier Science Publishers, 阿姆斯特丹Ruppel S, Merbach W (1995) Effects of different nitrogen sources on nitrogen fixation and bacterial growth of Pantoea agglomerans and Azospirillum sp in bacterial pure culture: an investigation using 15N2 incorporation and acetylene reduction measures. Microbiol Res 150:409–418
Ruppel S、Merbach W (1995) 不同氮源對 Pantoea agglomerans 與 Azospirillum sp.在純菌培養中固氮作用及細菌生長的影響:採用 15 N 2 標記與乙炔還原法的研究。Microbiol Res 150:409–418Ruppel S, Rühlmann J, Merbach W (2006) Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil 286:21–35
Ruppel S、Rühlmann J、Merbach W(2006)利用即時定量 PCR 與線上放射指紋圖譜技術對植物組織中細菌進行定量與定位分析。《植物與土壤》286:21-35Ruppel S, Krumbein A, Schreiner M (2008) Composition of the phyllospheric microbial populations on vegetable plants with different glucosinolate and carotenoid compositions. Microb Ecol 56:364–372
Ruppel S、Krumbein A、Schreiner M (2008) 不同芥子油苷與類胡蘿蔔素組成之蔬菜作物葉際微生物族群組成。Microb Ecol 56:364–372Schilling G, Gransee A, Deubel A, Lezovic G, Ruppel S (1998) Phosphorus availability, root exudates, and microbial activity in the rhizosphere. Z Pflanzen Bodenk 161:465–478
Schilling G、Gransee A、Deubel A、Lezovic G、Ruppel S (1998) 根際中的磷有效性、根系分泌物與微生物活性。Z Pflanzen Bodenk 161:465–478Schlaeppi K, Dombrowski N, Oter RG, van Themaat EVL, Schulze-Lefert P (2014) Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc Natl Acad Sci U S A 111:585–592
Schlaeppi K、Dombrowski N、Oter RG、van Themaat EVL、Schulze-Lefert P (2014) 阿拉伯芥近緣種中細菌根部微生物群的定量差異。《美國國家科學院院刊》111:585–592Scholz-Seidel C, Ruppel S (1992) Nitrogenase activities and phytohormone activities of Pantoea agglomerans in culture and their reflection in combination with wheat plants. Zentralblatt Für Mikrobiologie 147:319–328
Scholz-Seidel C, Ruppel S (1992) 培養條件下 Pantoea agglomerans 的固氮酶活性與植物激素活性及其與小麥植株結合後的表現。Zentralblatt Für Mikrobiologie 147:319–328Schreiner M, Krumbein A, Ruppel S (2009) Interaction between plants and bacteria: Glucosinolates and phyllospheric colonization of cruciferous vegetables by Enterobacter radicincitans DSM 16656. J Mol Microbiol Biotechnol 17:124–135
Schreiner M、Krumbein A、Ruppel S (2009) 植物與細菌間的交互作用:硫代葡萄糖苷與 Enterobacter radicincitans DSM 16656 對十字花科蔬菜葉際的定殖。J Mol Microbiol Biotechnol 17:124–135Schwachtje J, Karojet S, Thormahlen I, Bernholz C, Kunz S, Brouwer S, Schwochow M, Kohl K, van Dongen JT (2011) A naturally associated rhizobacterium of Arabidopsis thaliana induces a starvation-like transcriptional response while promoting growth. PLoS One 6:e29382
Schwachtje J, Karojet S, Thormahlen I, Bernholz C, Kunz S, Brouwer S, Schwochow M, Kohl K, van Dongen JT (2011) 一種阿拉伯芥自然伴生的根際細菌在促進生長的同時誘發類似飢餓狀態的轉錄反應。PLoS One 6:e29382Strehmel N, Böttcher C, Schmidt S, Scheel D (2014) Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 108:35–46
Strehmel N, Böttcher C, Schmidt S, Scheel D (2014) 阿拉伯芥根系分泌物中次級代謝物的分析。Phytochemistry 108:35–46Strehmel N, Monchgesang S, Herklotz S, Kruger S, Ziegler J, Scheel D (2016) Piriformospora indica stimulates root metabolism of Arabidopsis thaliana. Int J Mol Sci 17:1091
Strehmel N, Monchgesang S, Herklotz S, Kruger S, Ziegler J, Scheel D (2016) 印度梨形孢刺激擬南芥根部代謝作用。國際分子科學期刊 17:1091Sun HH, Wang L, Zhang BQ, Ma JH, Hettenhausen C, Cao GY, Sun GL, Wu JQ, Wu JS (2014) Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J Exp Bot 65:4305–4315
Sun HH, Wang L, Zhang BQ, Ma JH, Hettenhausen C, Cao GY, Sun GL, Wu JQ, Wu JS (2014) 東莨菪素是野生菸草中依賴茉莉酸信號途徑對抗鏈格孢菌的植物抗毒素。實驗植物學雜誌 65:4305–4315Taghavi S, van der Lelie D, Hoffman A, Zhang YB, Walla MD, Vangronsveld J, Newman L, Monchy S (2010) Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet 6
Taghavi S, van der Lelie D, Hoffman A, Zhang YB, Walla MD, Vangronsveld J, Newman L, Monchy S (2010) 植物生長促進內生細菌 Enterobacter sp. 638 之基因組定序。 PLoS Genet 6Tholl D (2015) Biosynthesis and biological functions of terpenoids in plants. In: Schrader J, Bohlmann J (eds) Biotechnology of Isoprenoids. Springer International Publishing, Cham
Tholl D (2015) 植物中萜類化合物的生物合成與生物功能。見:Schrader J, Bohlmann J (編) 《異戊二烯類生物技術》。Springer International Publishing, ChamVacheron J, Desbrosses G, Bouffaud M-L, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356
Vacheron J, Desbrosses G, Bouffaud M-L, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) 促進植物生長之根際細菌與根系功能。《植物科學前沿》4:356van de Mortel JE, de Vos RCH, Dekkers E, Pineda A, Guillod L, Bouwmeester K, van Loon JJA, Dicke M, Raaijmakers JM (2012) Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol 160:2173–2188
van de Mortel JE、de Vos RCH、Dekkers E、Pineda A、Guillod L、Bouwmeester K、van Loon JJA、Dicke M、Raaijmakers JM (2012) 根際細菌螢光假單胞菌 SS101 誘導擬南芥的代謝與轉錄組變化。植物生理學 160:2173–2188Vanholme R, Storme V, Vanholme B, Sundin L, Christensen JH, Goeminne G, Halpin C, Rohde A, Morreel K, Boerjan W (2012) A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell 24:3506–3529
Vanholme R、Storme V、Vanholme B、Sundin L、Christensen JH、Goeminne G、Halpin C、Rohde A、Morreel K、Boerjan W (2012) 阿拉伯芥木質素生物合成擾動反應的系統生物學觀點。植物細胞 24:3506–3529Verbon EH, Liberman LM (2016) Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci 21:218–229
Verbon EH, Liberman LM (2016) 有益微生物影響根系發育的內源性調控機制。植物科學趨勢 21:218–229Verhagen BWM, Glazebrook J, Zhu T, Chang HS, van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant-Microbe Interact 17:895–908
Verhagen BWM, Glazebrook J, Zhu T, Chang HS, van Loon LC, Pieterse CMJ (2004) 阿拉伯芥中根際細菌誘導系統抗性的轉錄組分析。Mol Plant-Microbe Interact 17:895–908Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vessey JK (2003) 促進植物生長之根際細菌作為生物肥料。Plant Soil 255:571–586Walker V, Bertrand C, Bellvert F, Moenne-Loccoz Y, Bally R, Comte G (2011) Host plant secondary metabolite profiling shows a complex, strain-dependent response of maize to plant growth-promoting rhizobacteria of the genus Azospirillum. New Phytol 189:494–506
Walker V, Bertrand C, Bellvert F, Moenne-Loccoz Y, Bally R, Comte G (2011) 宿主植物次級代謝物分析顯示玉米對固氮螺菌屬植物生長促進根際細菌具有複雜且菌株依賴性的反應。新植物學家 189:494–506Walker V, Couillerot O, Von Felten A, Bellvert F, Jansa J, Maurhofer M, Bally R, Moenne-Loccoz Y, Comte G (2012) Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomus consortium under field conditions. Plant Soil 356:151–163
Walker V、Couillerot O、Von Felten A、Bellvert F、Jansa J、Maurhofer M、Bally R、Moenne-Loccoz Y、Comte G (2012) 田間條件下接種固氮螺菌、假單胞菌與球囊黴菌聯合體對玉米幼苗根系次級代謝物含量的影響。植物與土壤 356:151–163Walter MH (2013) Role of carotenoid metabolism in the arbuscular mycorrhizal symbiosis. Molecular microbial ecology of the Rhizosphere. Wiley, Inc
Walter MH (2013) 類胡蘿蔔素代謝在叢枝菌根共生中的作用。《根際分子微生物生態學》。威利出版公司Walter MH, Floss DS, Strack D (2010) Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232:1–17
Walter MH、Floss DS、Strack D (2010) 脫輔基類胡蘿蔔素:賀爾蒙、菌根代謝物與芳香揮發物。《植物學》232 期:1-17 頁Wang YQ, Ohara Y, Nakayashiki H, Tosa Y, Mayama S (2005) Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol Plant-Microbe Interact 18:385–396
Wang YQ、Ohara Y、Nakayashiki H、Tosa Y、Mayama S (2005) 內生性植物促生根際細菌 Pseudomonas fluorescens FPT9601-T5 在阿拉伯芥中誘導基因表達譜的微陣列分析。Mol Plant-Microbe Interact 18:385–396Weston DJ, Pelletier DA, Morrell-Falvey JL, Tschaplinski TJ, Jawdy SS, Lu TY, Allen SM, Melton SJ, Martin MZ, Schadt CW, Karve AA, Chen JG, Yang XH, Doktycz MJ, Tuskan GA (2012) Pseudomonas fluorescens induces strain-dependent and strain-independent host plant responses in defense networks, primary metabolism, photosynthesis, and fitness. Mol Plant-Microbe Interact 25:765–778
Weston DJ、Pelletier DA、Morrell-Falvey JL、Tschaplinski TJ、Jawdy SS、Lu TY、Allen SM、Melton SJ、Martin MZ、Schadt CW、Karve AA、Chen JG、Yang XH、Doktycz MJ、Tuskan GA (2012) 螢光假單胞菌誘導宿主植物產生菌株依賴性與非依賴性之防禦網絡、初級代謝、光合作用及適應性反應。Mol Plant-Microbe Interact 25:765–778Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) 利用植物-微生物共生關係提升生物量生產與污染整治效益。生物技術趨勢 27:591–598Wintermans PCA, Bakker P, Pieterse CMJ (2016) Natural genetic variation in Arabidopsis for responsiveness to plant growth-promoting rhizobacteria. Plant Mol Biol 90:623–634
Wintermans PCA、Bakker P、Pieterse CMJ (2016) 阿拉伯芥對植物根際促生菌反應的自然遺傳變異。《植物分子生物學》90:623–634Witzel K, Gwinn-Giglio M, Nadendla S, Shefchek K, Ruppel S (2012) Genome sequence of Enterobacter radicincitans DSM16656T, a plant growth-promoting endophyte. J Bacteriol 194:5469
Witzel K, Gwinn-Giglio M, Nadendla S, Shefchek K, Ruppel S (2012) 植物促生內生菌 Enterobacter radicincitans DSM16656 T 之基因組定序。J Bacteriol 194:5469Witzel K, Hanschen FS, Schreiner M, Krumbein A, Ruppel S, Grosch R (2013) Verticillium suppression is associated with the glucosinolate composition of Arabidopsis thaliana leaves. PLoS One 8:e71877
Witzel K、Hanschen FS、Schreiner M、Krumbein A、Ruppel S、Grosch R (2013) 阿拉伯芥葉片中芥子油苷組成與黃萎病抑制之關聯性。《PLoS One》8:e71877Xu D, Hanschen FS, Witzel K, Nintemann SJ, Nour-Eldin HH, Schreiner M, Halkier BA (2016) Rhizosecretion of stele-synthesized glucosinolates and their catabolites requires GTR-mediated import in Arabidopsis. J Exp Bot 68:3205–3214
Xu D, Hanschen FS, Witzel K, Nintemann SJ, Nour-Eldin HH, Schreiner M, Halkier BA (2016) 中柱合成之硫代葡萄糖苷及其代謝產物的根部分泌需要阿拉伯芥中 GTR 媒介的轉運作用。實驗植物學雜誌 68:3205–3214Zachow C, Muller H, Tilcher R, Berg G (2014) Differences between the rhizosphere microbiome of Beta vulgaris ssp maritima - ancestor of all beet crops - and modern sugar beets. Front Microbiol 5:13
Zachow C, Muller H, Tilcher R, Berg G (2014) 所有甜菜作物祖先—濱海甜菜(Beta vulgaris ssp maritima)與現代糖用甜菜根際微生物組之差異。微生物學前沿 5:13Zhang Y, Ruyter-Spira C, Bouwmeester HJ (2015) Engineering the plant rhizosphere. Curr Opin Biotechnol 32:136–142
張 Y、Ruyter-Spira C、Bouwmeester HJ (2015) 植物根際工程。當代生物技術觀點 32:136–142
Acknowledgements 致謝
The technical assistance of Sabine Breitkopf, Andrea Jankowsky, Sylvia Krüger, Annett Platalla, Birgit Wernitz and Sieglinde Widiger is gratefully acknowledged. We thank Christoph Böttcher (Leibniz Institute of Plant Biochemistry, Germany) for the gift of leucylproline standard, Ellen Lagendjik (Leiden University, Netherlands) for provision of eGFP plasmid pMP4655, and Stefanie Döll (Leibniz Institute of Plant Biochemistry, Germany), Barbara Halkier and Deyang Xu (University of Copenhagen, Denmark) for transgenic Arabidopsis lines. This work was supported by the German Leibniz association (PAKT project ‘Chemical Communication in the Rhizosphere’, SAW-2011-IPB-3).
衷心感謝 Sabine Breitkopf、Andrea Jankowsky、Sylvia Krüger、Annett Platalla、Birgit Wernitz 和 Sieglinde Widiger 的技術協助。我們感謝 Christoph Böttcher(德國萊布尼茨植物生化研究所)惠贈亮氨醯脯氨酸標準品,Ellen Lagendjik(荷蘭萊頓大學)提供 eGFP 質體 pMP4655,以及 Stefanie Döll(德國萊布尼茨植物生化研究所)、Barbara Halkier 和 Deyang Xu(丹麥哥本哈根大學)提供轉基因擬南芥品系。本研究由德國萊布尼茨協會資助(PAKT 計畫「根際化學通訊」,SAW-2011-IPB-3)。
Additional information 補充資訊
Responsible Editor: Hans Lambers.
責任編輯:Hans Lambers。
Electronic supplementary material
電子補充材料
ESM 1
(DOCX 2052 kb) (DOCX 2052 kb)
ESM 2 電子補充材料 2
(DOCX 806 kb) (DOCX 806 kb)
ESM 3 電子補充材料 3
(DOCX 131 kb) (DOCX 131 kb)
ESM 4
(DOCX 17.4 kb) (DOCX 17.4 kb)
ESM 5 電子補充材料 5
(DOCX 16.3 kb) (DOCX 16.3 kb)
ESM 6 電子補充材料 6
(DOCX 17.6 kb) (DOCX 17.6 kb)
Rights and permissions 權利與權限
About this article 關於本文
Cite this article 引用本文
Witzel, K., Strehmel, N., Baldermann, S. et al.
Arabidopsis thaliana root and root exudate metabolism is altered by the growth-promoting bacterium Kosakonia radicincitans DSM 16656T
.
Plant Soil 419, 557–573 (2017). https://doi.org/10.1007/s11104-017-3371-1
Witzel, K.、Strehmel, N.、Baldermann, S. 等人。《促進生長的細菌 Kosakonia radicincitans DSM 16656T 改變擬南芥根部及根部分泌物代謝》。Plant Soil 419, 557–573 (2017)。https://doi.org/10.1007/s11104-017-3371-1
Received 已接收:
Accepted 已接受:
Published 發表:
Issue Date 出版日期:
DOI 數位物件識別碼: https://doi.org/10.1007/s11104-017-3371-1