Abstract 摘要
Neuroinflammation plays a crucial role in traumatic brain injury (TBI), contributing to both damage and recovery, yet no effective therapy exists to mitigate central nervous system (CNS) injury and promote recovery after TBI. In the present study, we found that nasal administration of an anti-CD3 monoclonal antibody ameliorated CNS damage and behavioral deficits in a mouse model of contusional TBI. Nasal anti-CD3 induced a population of interleukin (IL)-10-producing regulatory T cells (Treg cells) that migrated to the brain and closely contacted microglia. Treg cells directly reduced chronic microglia inflammation and regulated their phagocytic function in an IL-10-dependent manner. Blocking the IL-10 receptor globally or specifically on microglia in vivo abrogated the beneficial effects of nasal anti-CD3. However, the adoptive transfer of IL-10-producing Treg cells to TBI-injured mice restored these beneficial effects by enhancing microglial phagocytic capacity and reducing microglia-induced neuroinflammation. These findings suggest that nasal anti-CD3 represents a promising new therapeutic approach for treating TBI and potentially other forms of acute brain injury.
神经炎症在创伤性脑损伤(TBI)中起关键作用,既参与损伤过程也影响恢复进程,但目前尚无有效疗法能减轻中枢神经系统(CNS)损伤并促进 TBI 后恢复。本研究发现,在脑挫伤 TBI 小鼠模型中,鼻腔给予抗 CD3 单克隆抗体可改善 CNS 损伤和行为缺陷。鼻腔抗 CD3 治疗诱导产生了一群分泌白细胞介素(IL)-10 的调节性 T 细胞(T reg 细胞),这些细胞迁移至脑部并与小胶质细胞密切接触。T reg 细胞通过 IL-10 依赖性方式直接减轻慢性小胶质细胞炎症并调节其吞噬功能。在体内全局性或特异性阻断小胶质细胞的 IL-10 受体后,鼻腔抗 CD3 的治疗效果被消除。而将分泌 IL-10 的 T reg 细胞过继转移至 TBI 损伤小鼠体内,则通过增强小胶质细胞吞噬能力和减轻小胶质细胞介导的神经炎症,恢复了这些有益效应。这些发现表明鼻腔抗 CD3 疗法为治疗 TBI 及其他急性脑损伤提供了潜在的新型治疗策略。
Subject terms: Neuroimmunology, Inflammation, Microglia
主题词:神经免疫学、炎症、小胶质细胞
Nasal anti-CD3 therapy shows promise for treating traumatic brain injury by reducing neuroinflammation and aiding recovery in mice. It induces interleukin-10-producing regulatory T cells that enhance microglial phagocytic activity and reduce chronic inflammation, potentially aiding brain repair.
鼻腔抗 CD3 疗法通过减轻神经炎症和促进小鼠恢复,显示出治疗创伤性脑损伤的潜力。该疗法能诱导产生白细胞介素-10 的调节性 T 细胞,从而增强小胶质细胞的吞噬活性并减少慢性炎症,可能有助于脑部修复。
Main 主要
Traumatic brain injury (TBI) is a leading cause of death and disability, with both direct and indirect costs1–3. TBI is implicated in long-term morbidity, including motor deficits, cognitive decline and long-term medical comorbidities and neurodegeneration4–6. Current treatments primarily focus on early surgical intervention to limit hematoma expansion and supportive therapy; however, there are few pharmacological interventions to reduce long-term cognitive sequelae post-injury7–10. TBI induces a primary mechanical injury followed by a secondary biochemical and cellular response which contributes to neurological impairment11. Critically, neuroinflammation is one of the key mechanisms implicated in both the acute and the chronic phases of TBI12 and, as such, has been identified as an important and potentially modifiable driver of secondary injury in both animal and human studies13–19. TBI drives secondary injury via the activation of resident microglia, induction of cytokine release and recruitment of circulating monocytes or lymphocytes to the CNS, which further amplifies pathological inflammation11,20. Despite the clear clinical implications, no treatment specifically targets this neuroinflammatory process, partly as the result of the largely unknown molecular and cellular mechanisms that lead to neurological deficits after TBI20,21. Therefore, identifying new therapies to address chronic CNS inflammation after TBI represents an important unmet need.
创伤性脑损伤(TBI)是导致死亡和残疾的主要原因,会产生直接和间接成本 1–3 。TBI 与长期发病率相关,包括运动功能障碍、认知能力下降以及长期医学共病和神经退行性变 4–6 。当前的治疗主要侧重于通过早期手术干预限制血肿扩大和支持性治疗;然而,能减少损伤后长期认知后遗症的药物治疗手段十分有限 7–10 。TBI 首先引发原发性机械损伤,继而产生继发性生化和细胞反应,从而导致神经功能缺损 11 。关键的是,神经炎症是 TBI 急性和慢性阶段的关键机制之一 12 ,因此动物和人类研究均将其确定为继发性损伤的重要且具有潜在可调控性的驱动因素 13–19 。TBI 通过激活驻留小胶质细胞、诱导细胞因子释放以及招募循环单核细胞或淋巴细胞进入中枢神经系统,从而加剧病理性炎症反应,驱动继发性损伤 11,20 。 尽管其临床意义明确,但目前尚无专门针对这一神经炎症过程的治疗方法,部分原因是导致 TBI 后神经功能缺损的分子和细胞机制在很大程度上仍属未知 20,21 。因此,开发针对 TBI 后慢性中枢神经系统炎症的新疗法,是一项亟待满足的重要临床需求。
FoxP3+ regulatory T cells (Treg cells) represent a crucial subset of CD4+ T cells that modulate and limit adherent inflammatory responses22. There is a growing body of evidence showing that FoxP3+ Treg cells play a critical role in maintaining immune homeostasis and suppressing immune responses in several acute23,24 and chronic neurological diseases25,26. Experimentally, the depletion of FoxP3+ Treg cells in mice with controlled cortical impact (CCI) injuries leads to increased T cell infiltration, enhanced reactive astrogliosis and exacerbated motor deficits27. Conversely, expanding CD4+ FoxP3+ Treg cells through IL-2 treatment has been shown to improve outcomes in animal models of TBI28. In humans with TBI, the level of CD4+CD25+FoxP3+ Treg cells positively correlates with clinical outcomes29. Together, these studies suggest that inducing Treg cells is a promising approach for the treatment of TBI.
FoxP3+调节性 T 细胞(Treg 细胞)是 CD4+ T 细胞中至关重要的亚群,能够调节和限制持续性炎症反应。越来越多的证据表明,FoxP3+ Treg 细胞在维持免疫稳态和抑制多种急性及慢性神经系统疾病的免疫反应中发挥关键作用。实验研究表明,在控制性皮质撞击(CCI)损伤小鼠中清除 FoxP3+ Treg 细胞会导致 T 细胞浸润增加、反应性星形胶质细胞增生加剧以及运动功能障碍恶化。相反,通过 IL-2 治疗扩增 CD4+FoxP3+ Treg 细胞可改善 TBI 动物模型的预后。在人类 TBI 患者中,CD4+CD25+FoxP3+ Treg 细胞水平与临床预后呈正相关。这些研究共同表明,诱导 Treg 细胞是治疗 TBI 的一种有前景的方法。
The mucosal immune system is a unique tolerogenic organ that provides a physiological method for inducing Treg cells and is clinically appealing as a result of its apparent lack of toxicity. Our laboratory has investigated the induction of Treg cells by the nasal administration of anti-CD3 monoclonal antibody (aCD3 mAb) and has shown the ameliorating effects of nasal aCD3 in both autoimmune30 and CNS models of disease, including models of progressive multiple sclerosis (MS)31 and Alzheimer’s disease32. Based on this, we investigated whether nasal aCD3 could influence outcomes in relevant TBI models by modulating both systemic and local CNS immune responses.
黏膜免疫系统是一种独特的免疫耐受器官,可通过生理性方式诱导调节性 T 细胞(Treg),且因其明显无毒性而在临床上备受关注。我们实验室通过鼻腔给予抗 CD3 单克隆抗体(aCD3 mAb)诱导 Treg 细胞,并证实鼻腔给予 aCD3 在自身免疫性疾病和中枢神经系统疾病模型(包括进展型多发性硬化症(MS)和阿尔茨海默病模型)中均具有改善作用。基于此,我们研究了鼻腔给予 aCD3 是否可通过调节全身及局部中枢神经系统免疫反应来影响创伤性脑损伤(TBI)模型的预后。
Accordingly, we demonstrated that nasal aCD3 induces FoxP3+ Treg cells, which ameliorate TBI by modulating CNS innate immunity, enhancing microglia phagocytosis and improving neuropathological and behavioral outcomes post-injury in an IL-10-dependent manner. These findings highlight a new immune-based approach for treating TBI and potentially other types of acute brain injury.
因此,我们证实鼻内给予 aCD3 抗体可诱导 FoxP3 + T reg 细胞,这些细胞通过调节中枢神经系统先天免疫、增强小胶质细胞吞噬功能并以 IL-10 依赖性方式改善损伤后的神经病理学和行为学结局,从而缓解创伤性脑损伤。该研究结果揭示了一种治疗 TBI 及潜在其他类型急性脑损伤的新型免疫疗法。
Results 结果
Nasal aCD3 mAb improves cognitive and motor outcomes after TBI
鼻腔抗 CD3 单克隆抗体可改善创伤性脑损伤后的认知和运动功能
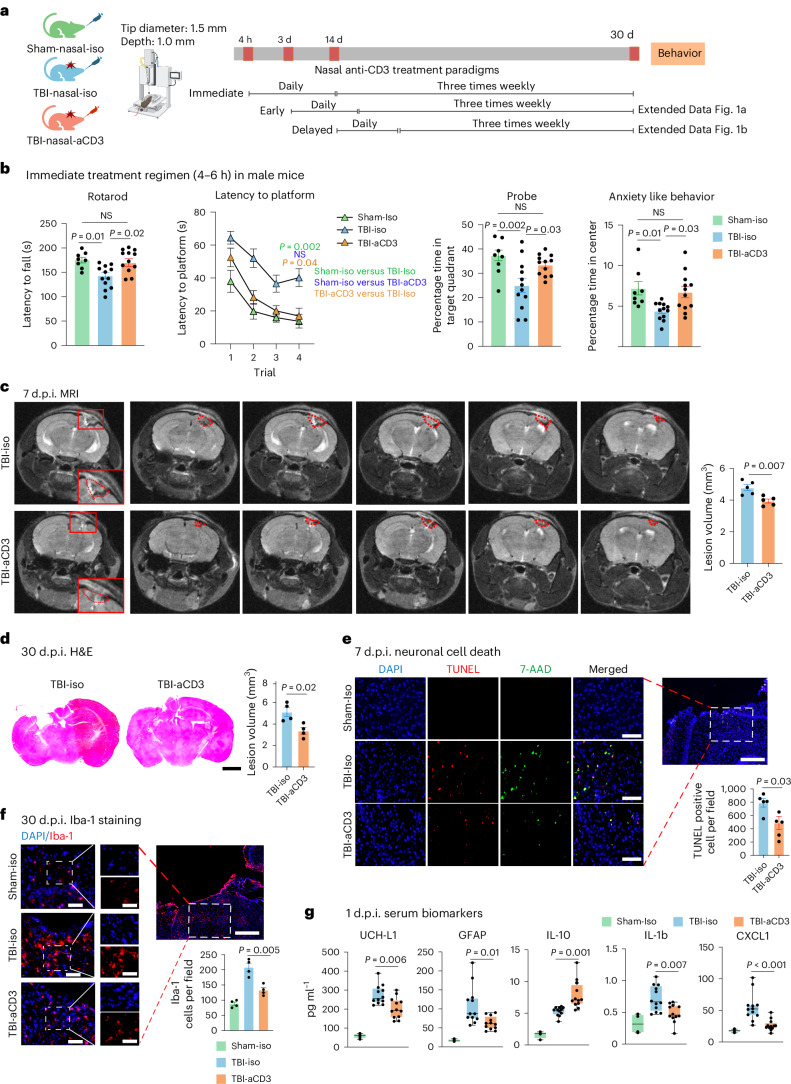
To investigate the therapeutic effect of nasal aCD3 in TBI, we employed the CCI model of TBI, which is known for its accuracy and reproducibility33, to recapitulate features of moderate-to-severe TBI features, including cerebral contusion, neuroinflammation, blood–brain barrier (BBB) dysfunction and long-term behavioral outcomes. We investigated the therapeutic effects of nasal aCD3 on motor and behavioral outcomes in male mice treated at different times after injury: immediate (4-6 h post-injury), early (3 d post-injury) and delayed (14 d post-injury) (Fig. 1a). Treatment was continued once daily for 7 d, then 3× weekly for up to 1 month after injury (Fig. 1a). In the immediate treatment group, we found improvement in motor function and coordination assessed by the rotarod test (Fig. 1b). Using the Morris water maze (MWM) test, TBI mice treated with aCD3 exhibited near-complete restoration of spatial memory and increased time spent in the target quadrant during the probe trial (Fig. 1b) compared with isotype-treated TBI mice. In addition, we found that male mice treated with immediate nasal aCD3 exhibited less anxious behavior, using the open field test. We found a similar beneficial effect with early treatment (3 d post-injury), except for anxiety (Extended Data Fig. 1a).
为探究鼻腔给予 aCD3 对创伤性脑损伤(TBI)的治疗效果,我们采用具有高度准确性和可重复性的 CCI 模型 33 来模拟中重度 TBI 特征,包括脑挫伤、神经炎症、血脑屏障(BBB)功能障碍及长期行为学改变。我们研究了不同时间窗(伤后立即[4-6 小时]、早期[3 天]和延迟期[14 天])给予鼻腔 aCD3 对雄性小鼠运动及行为学结局的影响(图 1a )。治疗方案为每日 1 次连续 7 天,之后每周 3 次持续至伤后 1 个月(图 1a )。在立即治疗组中,通过转棒测试发现运动功能和协调性改善(图 1b )。Morris 水迷宫(MWM)测试显示,与同型对照处理的 TBI 小鼠相比,aCD3 治疗组小鼠的空间记忆能力接近完全恢复,且在探测试验中目标象限停留时间显著延长(图 1b )。此外,通过旷场实验发现,立即鼻腔给予 aCD3 的雄性小鼠表现出更少的焦虑行为。 我们发现早期治疗(伤后 3 天)具有类似的有益效果,但焦虑症状除外(扩展数据图 1a )。
Fig. 1. Nasal aCD3 improves behavioral outcomes and ameliorates neuropathological outcomes in the CCI model of TBI.
图 1. 鼻腔给予 aCD3 改善 CCI 创伤性脑损伤模型的行为学结果并减轻神经病理学损伤
a, Experimental timeline schematic for treatment regimens (created with BioRender.com). b, Behavioral testing (rotarod, MWM test, probe trial, open field for anxiety-like behavior) in the immediate treatment group (sham-iso n = 8, TBI-iso n = 12, TBI-aCD3 n = 12). The MWM test was analyzed by two-factor, repeated-measure, two-way ANOVA (group × time) and others by one-way ANOVA with Tukey’s multiple comparisons. Data are shown as mean ± s.e.m. c, MRI lesion volume 7 d post-TBI (TBI-iso n = 5, TBI-aCD3 n = 5), analyzed by two-sided, unpaired Student’s t-test. Data are shown as mean ± s.e.m. The red dashes indicate the lesion area. d, Lesion volume from H&E-stained brain sections 30 d post-TBI measured with ImageJ (TBI-iso n = 4, TBI-aCD3 n = 4), analyzed by two-sided, unpaired Student’s t-test. Data are shown as mean ± s.e.m. Scale bars, 1,000 µm. e, Immunofluorescence of neuronal death 7 d post-TBI at pericontusional cortex (DAPI, blue; TUNEL, red; 7-AAD, green). Scale bars, 200 µm (500 µm for zoomed out). TUNEL-positive cells were quantified using ImageJ (TBI-iso n = 5, TBI-aCD3 n = 5) and analyzed using two-sided, unpaired Student’s t-test. Data are shown as mean ± s.e.m. f, Immunofluorescence of Iba-1 at 30 d post-TBI in pericontusional cortex (DAPI, blue; Iba-1, red). Scale bars, 200 µm (500 µm for zoomed out). Iba-1+ cells quantified using ImageJ (sham-iso n = 4, TBI-iso n = 4, TBI-aCD3 n = 4), analyzed by one-way ANOVA with Tukey’s multiple comparisons. Data are shown as mean ± s.e.m. g, Serum biomarkers 1 d post-TBI measured by Quanterix SiMoA and V-Plex proinflammatory assays (sham-iso n = 4, TBI-iso n = 12, TBI-aCD3 n = 12), analyzed by one-way ANOVA with Tukey’s multiple comparisons. Data are shown as box plots (min., max., interquartile range (IQR), median). d.p.i., d post-injury; NS, non-significant. All data represent biological replicates from two independent experiments.
a、治疗方案实验时间轴示意图(使用 BioRender.com 创建)。b、即时治疗组行为学测试(转棒试验、莫里斯水迷宫测试、探针测试、旷场焦虑样行为检测)(假手术组同型对照 n=8,TBI 组同型对照 n=12,TBI-aCD3 组 n=12)。莫里斯水迷宫采用双因素重复测量双因素方差分析(组别×时间),其余采用单因素方差分析结合 Tukey 多重比较。数据以均值±标准误表示。c、TBI 后 7 天 MRI 病灶体积(TBI 同型对照 n=5,TBI-aCD3 组 n=5),采用双侧非配对 t 检验分析。数据以均值±标准误表示。红色虚线标示病灶区域。d、TBI 后 30 天 H&E 染色脑切片病灶体积(使用 ImageJ 测量,TBI 同型对照 n=4,TBI-aCD3 组 n=4),采用双侧非配对 t 检验分析。数据以均值±标准误表示。比例尺:1,000 微米。e、TBI 后 7 天挫伤周边皮层神经元死亡免疫荧光检测(DAPI 蓝色,TUNEL 红色,7-AAD 绿色)。比例尺:200 微米(全景图 500 微米)。TUNEL 阳性细胞通过 ImageJ 定量(TBI 同型对照 n=5,TBI-aCD3 组 n=5)并采用双侧非配对 t 检验分析。 数据以均值±标准误表示。f 组:创伤性脑损伤后 30 天挫伤周围皮层 Iba-1 免疫荧光染色(DAPI 显蓝色,Iba-1 显红色)。比例尺:200 微米(缩略图 500 微米)。使用 ImageJ 软件定量 Iba-1 阳性细胞(假手术组同型对照 n=4,TBI 组同型对照 n=4,TBI-aCD3 组 n=4),采用单因素方差分析及 Tukey 多重比较法。数据以均值±标准误表示。g 组:采用 Quanterix SiMoA 和 V-Plex 促炎因子检测法测定创伤后 1 天血清生物标志物(假手术组同型对照 n=4,TBI 组同型对照 n=12,TBI-aCD3 组 n=12),采用单因素方差分析及 Tukey 多重比较法。数据以箱线图展示(最小值、最大值、四分位距、中位数)。d.p.i.:损伤后天数;NS:无统计学意义。所有数据均来自两次独立实验的生物学重复样本。
Extended Data Fig. 1. Nasal anti-CD3 improves behavioral outcomes at different treatment regimens and TBI severities.
扩展数据图 1. 鼻内抗 CD3 单抗通过不同治疗方案及 TBI 严重程度改善行为学结果
(a) Behavioral testing in the early treatment regimen for males of rotarod, Morris water maze, probe trial, and anxiety like behavior that is measured by the open field was assessed between (Sham-Iso n = 8, TBI-Iso n = 12, and TBI-aCD3 n = 12) groups in the early and (b) delayed treatment regimen for males and (c) immediate for females. Morris water maze analyzed by two-factor repeated measures two-way ANOVA (group x time); others by one-way ANOVA with Tukey’s multiple comparisons. Data shown as mean ± SEM. (d) Behavioral testing in the immediate treatment regimen for males in severe TBI (Depth: 1.5 mm, Diameter: 3.0 mm of the impact tip) of rotarod, Morris water maze, probe trial, and anxiety like behavior that is measured by the open field was assessed between (Sham-Iso n = 8, TBI-Iso n = 12, and TBI-aCD3 n = 12) groups in males and (e) females. Morris water maze analyzed by two-factor repeated measures two-way ANOVA (group x time); others by one-way ANOVA with Tukey’s multiple comparisons. Data shown as mean ± SEM. All data are biological replicates and are representative from two independent experiments. n.s. = non-significant.
(a) 早期治疗方案中雄性小鼠的行为学测试(转棒实验、Morris 水迷宫、探测试验及通过旷场实验测量的焦虑样行为)在(假手术组-Iso n=8、TBI-Iso n=12、TBI-aCD3 n=12)组间进行评估;(b) 延迟治疗方案中雄性小鼠及(c)雌性小鼠即刻治疗的行为学测试。Morris 水迷宫采用双因素重复测量双向方差分析(组别×时间),其余采用单因素方差分析结合 Tukey 多重比较。数据以均值±标准误表示。(d) 严重 TBI 雄性小鼠(撞击头深度 1.5mm/直径 3.0mm)即刻治疗方案中的转棒实验、Morris 水迷宫、探测试验及旷场焦虑样行为测试在(假手术组-Iso n=8、TBI-Iso n=12、TBI-aCD3 n=12)组间进行;(e) 雌性小鼠相应测试。Morris 水迷宫采用双因素重复测量双向方差分析(组别×时间),其余采用单因素方差分析结合 Tukey 多重比较。数据以均值±标准误表示。所有数据均为生物学重复样本,结果来自两次独立实验。n.s.表示无统计学显著性。
We then investigated the effect of delayed (14 d post-injury) nasal aCD3 treatment on behavioral outcomes in male mice and found no improvement in the treated the group compared with the TBI-iso control (Extended Data Fig. 1b). In addition to male mice, we investigated the effects of immediate nasal aCD3 treatment on TBI in female mice. We did not find notable differences in motor or spatial memory testing between the groups after moderate CCI. As reported in the literature, female rodents may outperform males in behavioral tasks after brain injury34–36. Thus, we were unable to demonstrate the beneficial effect of nasal aCD3 treatment in female mice at this injury level (Extended Data Fig. 1c).
随后,我们研究了延迟(伤后 14 天)鼻腔给予 aCD3 治疗对雄性小鼠行为学结果的影响,发现与 TBI-iso 对照组相比,治疗组未见改善(扩展数据图 1b )。除雄性小鼠外,我们还考察了即刻鼻腔 aCD3 治疗对雌性小鼠 TBI 的影响。在中度 CCI 后,我们未发现各组间在运动或空间记忆测试中存在显著差异。文献报道显示,脑损伤后雌性啮齿类动物在行为学任务中可能优于雄性 34–36 。因此,我们未能在此损伤程度下证明鼻腔 aCD3 治疗对雌性小鼠的获益效应(扩展数据图 1c )。
We then investigated the effect of nasal aCD3 treatment on behavioral outcomes in a more severe form of TBI (3 mm tip diameter and 1.5 mm depth) in both male and female mice. We found improvement in motor function and coordination in the TBI-aCD3 group when compared with TBI-iso control in both sexes (Extended Data Fig. 1d,e). There was partial restoration in spatial memory and increased time spent in the target quadrant during the probe trial in the nasal aCD3-treated mice compared with TBI-iso control in both sexes. Male mice treated with nasal aCD3 exhibited less anxiety-like behavior after severe TBI, but we were unable to assess the beneficial effects of nasal aCD3 in female mice because they did not develop an anxiety phenotype after severe TBI (Extended Data Fig. 1d,e). These data clearly demonstrate that nasal aCD3 improves behavioral outcomes in moderate and severe CCI-induced TBI, with a greater effect being observed in male mice.
随后,我们研究了鼻腔给予 aCD3 抗体治疗对雌雄两性小鼠更严重型 TBI(撞击头端直径 3 毫米、深度 1.5 毫米)行为学结局的影响。与 TBI-iso 对照组相比,两性 TBI-aCD3 治疗组均表现出运动功能和协调能力的改善(扩展数据图 1d,e )。鼻腔 aCD3 治疗组小鼠的空间记忆得到部分恢复,在探测试验中停留在目标象限的时间较 TBI-iso 对照组延长。在严重 TBI 后,鼻腔 aCD3 治疗的雄性小鼠表现出更少的焦虑样行为,但由于雌性小鼠在严重 TBI 后未出现焦虑表型,我们无法评估鼻腔 aCD3 对雌鼠的改善作用(扩展数据图 1d,e )。这些数据明确表明,鼻腔 aCD3 能改善中重度 CCI 诱导型 TBI 的行为学结局,且在雄性小鼠中观察到的疗效更为显著。
Nasal aCD3 mAb ameliorates TBI neuropathology
鼻腔给予 aCD3 单抗改善创伤性脑损伤神经病理学改变
TBI induces BBB disruption, edema, neuronal death and tissue loss, in addition to the increased production of inflammatory mediators and gliosis20. Therefore, we assessed the effects of nasal aCD3 on these neuropathological outcomes (Fig. 1a). We first measured the parenchymal lesion volume in sham-iso, TBI-aCD3 and TBI-iso groups at 7 d post-injury, using 3-tesla magnetic resonance imaging (MRI). We found a reduction in lesion volume in the nasal aCD3-treated group compared with TBI-iso controls (Fig. 1c and Extended Data Fig. 2a). We also measured lesion volume at 1 month post-CCI using hematoxylin and eosin (H&E) staining and, consistent with the MRI, we found a reduction in lesion volume in TBI-aCD3 mice compared with the TBI-iso control (Fig. 1d). Nasal aCD3 also improved BBB integrity at 3 d post-injury compared with the TBI-iso control (Extended Data Fig. 2b). The percentage of brain edema in the ipsilateral and contralateral hemispheres at 3 d post-CCI was reduced in the ipsilateral hemisphere for the TBI-aCD3 group compared with the TBI-iso control (Extended Data Fig. 2c). CCI increased cell death, as measured by TUNEL staining at 7 d after brain injury (Fig. 1e), which was reduced in nasal aCD3 treatment compared with TBI-iso controls. Consistent with previous reports, we found that CCI was associated with an increase in microglia or macrophage activation (Iba-1 staining) at 30 d post-injury compared with sham-iso controls (Fig. 1f)20,37. We found that there was a reduction in microgliosis at 30 d post-injury in male mice in both the immediate and the early nasal aCD3 groups (Fig. 1f and Extended Data Fig. 2d). Of note, TBI has also been associated with changes in serum biomarkers38 and we found a reduction in several TBI serum biomarkers in the immediate nasal aCD3 group, including glial fibrillary acidic protein (GFAP), UCH-L1 and the inflammatory cytokines IL-1b and CXCL1 versus controls. It is interesting that we also found an increase in the anti-inflammatory cytokine IL-10 in the immediate nasal aCD3 group versus control animals (Fig. 1g). These markers could be used to assess the efficacy of treatment in humans. As in male mice, we found that nasal aCD3 reduced lesion volume (Extended Data Fig. 2e) and microgliosis at the lesion site in female mice after severe TBI (Extended Data Fig. 2f). These data clearly demonstrate that the behavioral improvements observed in TBI mice treated with nasal aCD3 are associated with enhanced tissue integrity, as indicated by neuropathology and serum biomarkers.
创伤性脑损伤(TBI)除引发炎症介质增加和神经胶质增生外,还会导致血脑屏障破坏、水肿、神经元死亡及组织缺损 20 。为此,我们评估了鼻腔给予 aCD3 抗体对这些神经病理学结局的影响(图 1a )。首先采用 3 特斯拉磁共振成像(MRI)检测假手术组、TBI-aCD3 治疗组和 TBI 对照组在损伤后 7 天的脑实质病灶体积,发现鼻腔 aCD3 治疗组的病灶体积较 TBI 对照组显著减小(图 1c 及扩展数据图 2a )。通过苏木精-伊红(H&E)染色检测损伤后 1 个月的病灶体积,结果与 MRI 一致:TBI-aCD3 组小鼠的病灶体积小于 TBI 对照组(图 1d )。与 TBI 对照组相比,鼻腔 aCD3 在损伤后 3 天还能改善血脑屏障完整性(扩展数据图 2b )。在损伤后 3 天,TBI-aCD3 组小鼠损伤同侧大脑半球的脑水肿比例较 TBI 对照组降低,而对侧半球无显著差异(扩展数据图 2c )。通过 TUNEL 染色检测发现,控制性皮质撞击伤(CCI)在脑损伤后 7 天会加剧细胞死亡(图 1e ),与 TBI-iso 对照组相比,鼻内 aCD3 治疗组该指标降低。与既往报道一致,我们发现 CCI 损伤后 30 天小胶质细胞/巨噬细胞活化标志物(Iba-1 染色)较假手术组升高(图 1f ) 20,37 。值得注意的是,在雄性小鼠中,无论是即刻还是早期鼻内 aCD3 治疗组,损伤后 30 天的小胶质细胞增生均有所减少(图 1f 及扩展数据图 2d )。需特别指出的是,TBI 还与血清生物标志物改变相关 38 ,我们在即刻鼻内 aCD3 治疗组观察到多个 TBI 血清标志物水平降低,包括胶质纤维酸性蛋白(GFAP)、UCH-L1 以及炎症细胞因子 IL-1β和 CXCL1。有趣的是,与对照组相比,即刻鼻内 aCD3 治疗组抗炎细胞因子 IL-10 水平升高(图 1g ),这些标志物或可用于评估人类治疗效果。与雄性小鼠结果类似,我们发现鼻内 aCD3 能减少雌性小鼠严重 TBI 后的病灶体积(扩展数据图 2e )及病灶区小胶质细胞增生(扩展数据图 2f )。 这些数据清楚地表明,经鼻腔 aCD3 治疗的创伤性脑损伤小鼠所表现出的行为改善与组织完整性增强相关,神经病理学检查和血清生物标志物检测均证实了这一点。
Extended Data Fig. 2. Nasal anti-CD3 ameliorates pathological outcomes at different treatment regimens and TBI severities.
扩展数据图 2. 鼻腔给予抗 CD3 单抗通过不同治疗方案改善不同程度创伤性脑损伤的病理学结局
(a) Unedited 3-Tesla serial images Magnetic resonance imaging (MRI) taken 7 days post-TBI of (Fig. 1c). (b) Dextran 70-kDa (Green) for measurement of blood-brain barrier permeability between the groups (3days post-TBI). Scale bars are 1000 um. Data is shown as mean ± SEM, (Sham-Iso n = 3, TBI-Iso n = 4, and TBI-aCD3 n = 4) and analyzed by one-way ANOVA with Tukey’s multiple comparisons. (c) Brain edema was analyzed on day 3 post-TBI and % water content was measured between the ipsilateral and contralateral hemispheres. Data shown as mean ± SEM and n = 3-4 mice/group were used. Data was analyzed by two-sided unpaired Student’s t-test. (d) Immunofluorescence staining of Iba-1 30-days post-TBI for early treatment in males at the peri-contusional cortex for DAPI (blue) and Iba-1 (red). Scale bars are 250 um. Five sections of each sample were prepared and the area around the contusion was captured and the number of Iba-1 positive cells around the contusion were quantified by Image J. Data shown as mean ± SEM and n = 5 mice/group were used. Data was analyzed by two-sided unpaired Student’s t-test. (e) Brain sections were stained with DAPI (blue) at 30 days post severe TBI in females and lesion volume was measured by image J software. Data shown as mean ± SEM and n = 4 mice/group were used. Data was analyzed by two-sided unpaired Student’s t-test. Scale bars are 1000 um. (f) Immunofluorescence staining of Iba-1 30 days post severe TBI in females in the at the peri-contusional cortex for DAPI (blue) and Iba-1 (red). Scale bars are 250 um. Five sections of each sample were prepared and the area around the contusion was captured and the number of Iba-1 positive cells around the contusion were quantified by Image J. Data shown as mean ± SEM and n = 4 mice/group were used. Data was analyzed by two-sided unpaired Student’s t-test. All data are biological replicates and are representative from two independent experiments. n.s. = non-significant. DPI indicates Days Post Injury.
(a) 创伤性脑损伤(TBI)后 7 天获取的 3 特斯拉磁共振成像(MRI)连续图像(图 1c )。(b) 采用 70-kDa 右旋糖酐(绿色)测量各组间血脑屏障通透性(TBI 后 3 天)。比例尺为 1000 微米。数据以均值±标准误表示(假手术组-Iso n=3,TBI-Iso 组 n=4,TBI-aCD3 组 n=4),采用单因素方差分析及 Tukey 多重比较法进行统计分析。(c) TBI 后第 3 天分析脑水肿情况,并测量损伤侧与对侧大脑半球的水含量百分比。数据以均值±标准误表示,每组使用 3-4 只小鼠。采用双尾非配对 Student t 检验进行数据分析。(d) 雄性小鼠早期治疗组在 TBI 后 30 天进行 Iba-1 免疫荧光染色(损伤周边皮层),DAPI(蓝色)和 Iba-1(红色)标记。比例尺为 250 微米。每组制备 5 个切片,捕获挫伤周边区域并通过 Image J 软件定量挫伤周边 Iba-1 阳性细胞数量。数据以均值±标准误表示,每组使用 5 只小鼠。采用双尾非配对 Student t 检验进行数据分析。 (e) 雌性小鼠严重 TBI 后 30 天,脑切片经 DAPI 染色(蓝色显示),采用 Image J 软件测量病灶体积。数据以均值±标准误表示,每组 n=4 只小鼠。采用双侧非配对 Student t 检验进行数据分析。比例尺为 1000 微米。(f) 雌性小鼠严重 TBI 后 30 天,在挫伤周围皮层进行 Iba-1 免疫荧光染色,显示 DAPI(蓝色)和 Iba-1(红色)。比例尺为 250 微米。每个样本制备 5 张切片,捕获挫伤周围区域并使用 Image J 定量挫伤周围 Iba-1 阳性细胞数量。数据以均值±标准误表示,每组 n=4 只小鼠。采用双侧非配对 Student t 检验进行数据分析。所有数据均为生物学重复,结果来自两次独立实验。n.s.表示无统计学意义。DPI 指损伤后天数。
Nasal aCD3 mAb expands central and peripheral Treg cells after TBI
鼻腔给予抗 CD3 单克隆抗体(aCD3 mAb)在创伤性脑损伤(TBI)后促进中枢及外周调节性 T 细胞(Treg)扩增
TBI is associated with major changes in the cellular kinetics of both resident and infiltrating cells, which contribute to brain injury. Therefore, to characterize the effect of nasal aCD3 on TBI, we investigated the temporal roles of specific immune cell populations post-injury20. We performed flow cytometry analysis on immune cells isolated from cervical lymph nodes (cLNs), meninges and the ipsilateral hemisphere at multiple time points post-TBI. We found that the immediate treatment of nasal aCD3 increased the percentage of total CD4+ T cells and CD4+FoxP3+ Treg cells in cLNs at 1 d (Extended Data Fig. 3a) and increased the total number of those in the meninges at 2 d post-injury (Fig. 2a,b). Nasal aCD3 also expanded the total number of CD4+ T cells in the brain at 3 and 7 d post-injury and increased CD4+FoxP3+ Treg cells in the first 30 d post-injury compared with TBI isotype controls (Fig. 2a,b and Extended Data Figs. 3a and 4a). Of note, we did not observe an increase in CD4+ T cells expressing latency-associated peptide (LAP), a membrane-bound transforming growth factor β (TGF-β) compared with the TBI-iso group (Fig. 2c and Extended Data Figs. 3b and 4a). Thus, nasal aCD3 expands Treg cells to control neuroinflammation after TBI.
创伤性脑损伤(TBI)会导致驻留细胞和浸润细胞的细胞动力学发生重大变化,这些变化加剧了脑损伤。因此,为明确鼻腔给予 aCD3 抗体对 TBI 的作用机制,我们研究了损伤后特定免疫细胞亚群的时序性变化。通过流式细胞术分析 TBI 后多个时间点从颈淋巴结(cLNs)、脑膜及损伤同侧大脑半球分离的免疫细胞,发现鼻腔 aCD3 即时治疗可使损伤后 1 天 cLNs 中总 CD4+T 细胞及 CD4+FoxP3+T 细胞比例增加(扩展数据图),并在损伤后 2 天增加脑膜中这些细胞的总数(图)。与 TBI 同型对照组相比,鼻腔 aCD3 还使损伤后 3 天和 7 天脑内 CD4+T 细胞总数扩增,并在损伤后 30 天内持续增加 CD4+FoxP3+T 细胞数量(图及扩展数据图)。值得注意的是,与 TBI-iso 组相比,我们未观察到表达潜伏相关肽(LAP,一种膜结合型转化生长因子β)的 CD4+T 细胞增加(图。 2c 及扩展数据图 3b 和 4a )。因此,鼻腔给予 aCD3 可通过扩增 T reg 细胞来调控创伤性脑损伤后的神经炎症反应。
Extended Data Fig. 3. Nasal anti-CD3 ameliorates adaptive immune response following TBI.
扩展数据图 3. 鼻腔抗 CD3 治疗改善创伤性脑损伤后的适应性免疫应答。
(a) Flow cytometry analysis and quantification of CD4 + T cells, CD4+FoxP3 + and (b) CD8 + T cells, CD4 + LAP + , Th1, and Th17 at 1,3,7,14, and 30 days post TBI and nasal anti-CD3 treatment in the cervical lymph nodes, meninges, and Ipsilateral brain hemisphere. (Sham-Iso n = 4, TBI-Iso n = 6, TBI-aCD3 n = 6). Data shown as mean ± SEM and analyzed by one-way ANOVA with Tukey’s multiple comparisons for every individual timepoint (c) Flow cytometry analysis and quantification of neutrophils, monocytes, classical monocytes (7 days post-TBI), and NK cells at 1,3,7,14, and 30 days post TBI and nasal anti-CD3 treatment in Ipsilateral brain hemisphere. (Sham-Iso n = 4, TBI-Iso n = 6, TBI-aCD3 n = 6). Data shown as mean ± SEM and analyzed by one-way ANOVA with Tukey’s multiple comparisons for every individual timepoint. All data are biological replicates and are representative from two independent experiments.
(a) 流式细胞术分析及定量检测创伤性脑损伤(TBI)和鼻腔抗 CD3 单抗治疗后 1、3、7、14 和 30 天时颈淋巴结、脑膜及损伤侧大脑半球中 CD4+T 细胞、CD4+FoxP3+细胞、(b) CD8+T 细胞、CD4+LAP+细胞、Th1 和 Th17 细胞。(假手术组-Iso n=4,TBI-Iso 组 n=6,TBI-aCD3 组 n=6)。数据以均值±标准误表示,采用单因素方差分析及 Tukey 多重比较法对各时间点进行统计分析。(c) 流式细胞术分析及定量检测 TBI 和鼻腔抗 CD3 单抗治疗后 1、3、7、14 和 30 天时损伤侧大脑半球中性粒细胞、单核细胞、经典单核细胞(TBI 后 7 天)及 NK 细胞。(假手术组-Iso n=4,TBI-Iso 组 n=6,TBI-aCD3 组 n=6)。数据以均值±标准误表示,采用单因素方差分析及 Tukey 多重比较法对各时间点进行统计分析。所有数据均为生物学重复样本,结果代表两次独立实验。
Fig. 2. Nasal aCD3 expands FoxP3 Treg cells and modulates the adaptive immune response.
图 2. 鼻腔给予 aCD3 可扩增 FoxP3 T reg 细胞并调节适应性免疫应答。
a,b, Flow cytometry analysis and quantification of CD4+ (a) and CD4+FoxP3+ (b) Treg cells in the meninges and ipsilateral hemisphere at 1, 3, 7, 14 and 30 d (D) after TBI and treatment. c, Quantification of CD4+ subsets at the same time points. d, Analysis of CD11b+-infiltrated cells across these intervals. Groups included sham-iso (n = 4), TBI-iso (n = 6) and TBI-aCD3 (n = 6). Data are analyzed by one-way ANOVA with Tukey’s multiple comparisons for every individual timepoint. Data are shown as mean ± s.e.m. representing biological replicates from two independent experiments per timepoint for a–d. e, Immunofluorescence of meninges (2 d post-TBI) and brain (7 d post-TBI) samples from FoxP3-GFP mice with DAPI (blue), CD3 (pink), FoxP3 (green). Scale bars, 100 µm. IHC, immunohistochemistry. f, PCA plot of brain and blood Treg cells 7 d post-TBI. Brain and blood samples are pools of 5 mice and sham-iso brain samples are pools of 20 mice. Due to the low number of FoxP3⁺ cells recruited to the brain and ethical considerations, we limited the study to two biological replicates, following practices from previous studies in the field23. Despite this limitation, the consistent and robust results observed support the validity of our findings. g,h, Heatmaps of DEGs from blood (g) and brain (h) Treg cells at 7 d post-TBI using DESeq2 (FDR-corrected P < 0.05, n = 2 pooled samples per group). i, GSEA of GOBP 7 d post-TBI for brain Treg cells. The asterisks indicate enriched terms (q < 0.05). NES, normalized enrichment score. j, IPA analysis of DEGs from brain Treg cells in TBI-aCD3 versus TBI-iso using DESeq2 analysis (two-sided Wald’s test, FDR-corrected P < 0.05). One-sided Fisher’s exact test was used: *P < 0.05, **P < 0.01, ***P < 0.001. Results with FDR-corrected P < 0.05 were selected. k, Predicted upstream regulators using IPA for TBI-aCD3 versus TBI-iso. l, Quantification of FoxP3+IL-10+ Treg cells in the ipsilateral hemisphere 7 d post-TBI. Groups included sham-iso (n = 4), TBI-iso (n = 6) and TBI-aCD3 (n = 6). Data are shown as box plots (min., max., IQR, median), analyzed by one-way ANOVA with Tukey’s multiple comparisons. Data are from biological replicates and represent two independent experiments.
a,b,流式细胞术分析及定量检测创伤性脑损伤(TBI)后 1、3、7、14 和 30 天(D)脑膜与损伤同侧大脑半球中 CD4 + (a)和 CD4 + FoxP3 + (b)T reg 细胞。c,相同时间点 CD4 + 亚群的定量分析。d,各时间点 CD11b + 阳性浸润细胞分析。实验分组包括假手术同型对照组(n=4)、TBI 同型对照组(n=6)和 TBI-aCD3 治疗组(n=6)。数据采用单因素方差分析(ANOVA)结合 Tukey 多重比较法对各时间点进行统计学处理。a-d 数据以均值±标准误表示,每个时间点包含两项独立实验的生物学重复。e,FoxP3-GFP 小鼠脑膜(TBI 后 2 天)和脑组织(TBI 后 7 天)样本的免疫荧光染色:DAPI(蓝)、CD3(粉)、FoxP3(绿)。比例尺 100µm。IHC:免疫组织化学。f,TBI 后 7 天脑组织与血液 T reg 细胞的主成分分析(PCA)图。脑组织和血液样本为 5 只小鼠混合样本,假手术组脑样本为 20 只小鼠混合样本。鉴于脑内募集的 FoxP3⁺细胞数量较少及伦理考量,本研究遵循该领域既往研究规范,仅进行两项生物学重复实验 23 。 尽管存在这一局限性,我们观察到的持续且稳健的结果支持研究发现的可靠性。图 g、h 分别显示 TBI 后 7 天通过 DESeq2 分析获得的血液(g)和脑组织(h)T reg 细胞差异表达基因热图(FDR 校正 P 值<0.05,每组 n=2 混合样本)。图 i 为 TBI 后 7 天脑组织 T reg 细胞的 GOBP 基因集富集分析,星号标注显著富集条目(q 值<0.05)。NES 表示标准化富集分数。图 j 通过 IPA 分析比较 TBI-aCD3 组与 TBI-iso 组脑组织 T reg 细胞差异基因(采用 DESeq2 双尾 Wald 检验,FDR 校正 P 值<0.05),单尾 Fisher 精确检验标注: * P<0.05, ** P<0.01, *** P<0.001,筛选 FDR 校正 P 值<0.05 的结果。图 k 展示 IPA 预测的 TBI-aCD3 组与 TBI-iso 组上游调控因子。图 l 定量分析 TBI 后 7 天损伤同侧半球 FoxP3 + IL-10 + T reg 细胞数量,实验分组包括假手术-iso 组(n=4)、TBI-iso 组(n=6)和 TBI-aCD3 组(n=6)。数据以箱线图呈现(最小值、最大值、四分位距、中位数),采用单因素方差分析结合 Tukey 多重比较检验。所有数据均来自生物学重复样本并代表两次独立实验。
Extended Data Fig. 4. Gating strategy of different immune cells in TBI.
扩展数据图 4. TBI 中不同免疫细胞的门控策略。
(a) Gating strategy used to identify the different T-cell substypes. (b) Gating strategies used to identify different CD11b+ infiltrating cells and (c) CD11b+Ly6Chi monocytes.
(a) 用于识别不同 T 细胞亚群的设门策略。(b) 用于识别不同 CD11b+浸润细胞的设门策略及(c) CD11b+Ly6Chi 单核细胞的设门策略。
Nasal aCD3 mAb modulates the innate and adaptive immune response after TBI
鼻腔给予 aCD3 单抗可调节 TBI 后的先天性与适应性免疫应答
Nasal aCD3 also reduced the number of CD8+ cells at 14 and 30 d and helper T cells (TH1 and TH17 cells) at day 30 post-injury (Fig. 2c and Extended Data Figs. 3b and 4a). In addition, we found a significant reduction in neutrophil recruitment at day 1, monocytes at day 7 and natural killer cells at days 1 and 14 after CCI in the nasal aCD3 group compared with the TBI-iso controls (Fig. 2d and Extended Data Figs. 3c and 4b,c).
鼻腔给予 aCD3 还显著减少了伤后 14 天和 30 天的 CD8 + 细胞数量,以及伤后 30 天的辅助性 T 细胞(T H 1 和 T H 17 细胞)(图 2c 及扩展数据图 3b 和 4a )。此外,与 TBI 对照组相比,鼻腔 aCD3 治疗组在 CCI 后第 1 天中性粒细胞浸润、第 7 天单核细胞浸润以及第 1 天和第 14 天的自然杀伤细胞数量均出现显著减少(图 2d 及扩展数据图 3c 和 4b,c )。
Treg cells induced by nasal aCD3 have a unique immunomodulatory profile
鼻腔给予 aCD3 诱导产生的 T 细胞具有独特的免疫调节特性
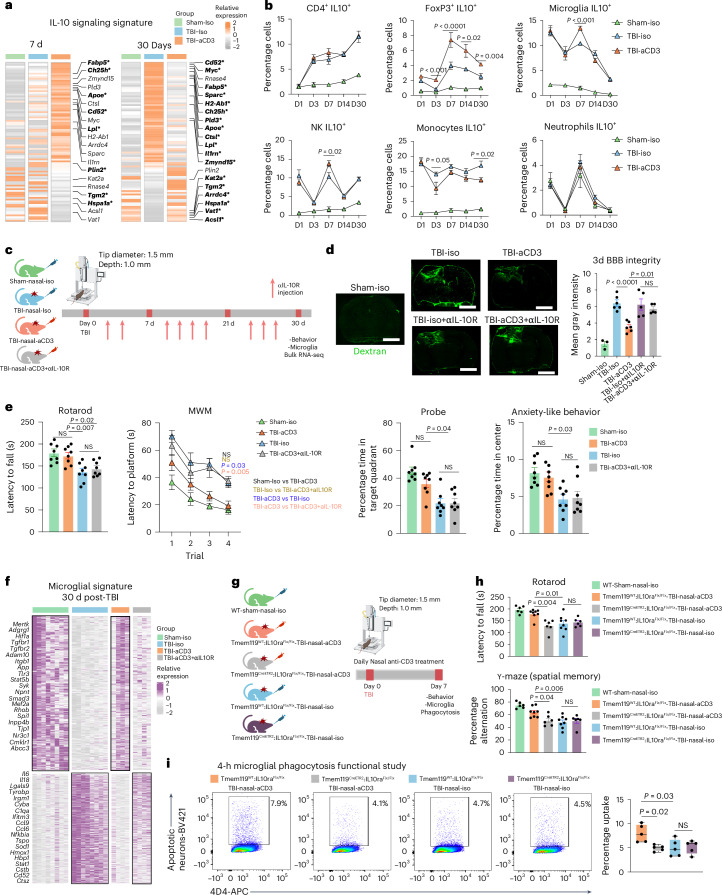
As shown in Fig. 2b, nasal aCD3 increased CD4+FoxP3+ Treg cells in the first 30 d post-injury compared with TBI isotype controls (Fig. 2b). To elucidate the mechanisms whereby aCD3-induced Treg cells may have contributed to post-TBI recovery, we performed RNA sequencing (RNA-seq) analysis on CD4+FoxP3(GFP)+ Treg cells isolated from both the pericontusional brain tissue and blood of the sham and injured mice 7 d after CCI (Fig. 2f–k, Extended Data Fig. 5a and Supplementary Table 1). Principal component analysis (PCA) showed that the transcriptomic profile of brain FoxP3 Treg cells was markedly different from blood FoxP3 Treg cells after TBI (Fig. 2f). Consistent with recent reports23, we found increased expression of multiple immunomodulatory and trophic factor genes (Il10, Spp1, Gas6, Igf1, Dab2, Lif, Areg, Il1r2, Irf8, Osm, Tgfa, Ccl8, and Hmox1) in brain-infiltrating Treg cells from TBI mice compared with blood Treg cells from sham mice (Extended Data Fig. 5b).
如图 2b 所示,与 TBI 同型对照组相比,鼻腔给予 aCD3 在损伤后 30 天内增加了 CD4 + FoxP3 + T reg 细胞数量(图 2b )。为阐明 aCD3 诱导的 T reg 细胞促进 TBI 后恢复的机制,我们在 CCI 术后 7 天对假手术组和损伤组小鼠挫伤周边脑组织及血液中分离的 CD4 + FoxP3(GFP) + T reg 细胞进行了 RNA 测序分析(图 2f–k 、扩展数据图 5a 及补充表 1 )。主成分分析(PCA)显示,TBI 后脑部 FoxP3 T reg 细胞的转录组特征与血液 FoxP3 T reg 细胞存在显著差异(图 2f )。与近期研究报道一致 23 ,我们发现相较于假手术组小鼠血液 T reg 细胞,TBI 小鼠脑浸润 T reg 细胞中多种免疫调节及神经营养因子基因(Il10、Spp1、Gas6、Igf1、Dab2、Lif、Areg、Il1r2、Irf8、Osm、Tgfa、Ccl8 和 Hmox1)表达上调(扩展数据图 5b )。
Extended Data Fig. 5. Nasal anti-CD3 induces a unique immune modulatory signature in FoxP3 Tregs.
扩展数据图 5. 鼻腔给予抗 CD3 单抗可诱导 FoxP3 调节性 T 细胞产生独特的免疫调节特征
(a) Gating strategy used to identify and sort CD4+FoxP3(GFP)+ from the ipsilateral hemisphere of the brain and blood. (b) Overlap in differentially expressed genes in injured brain vs. sham blood Treg cells after injury with another study investigating the transcriptomic effects of stroke in brain vs. blood Treg cells23. (c) Selected top predicted regulators using IPA based on DEGs in TBI-aCD3 vs. TBI-Iso blood Treg cells at 7 days post-TBI identified using DESeq2 analysis (two-sided Wald test, FDR-corrected P < 0.05). One-sided Fisher’s exact test. * P < 0.05, ** P < 0.01, *** P < 0.001. Results with FDR-corrected P < 0.05 were selected. (d) Predicted upstream regulator using IPA analysis based on DEGs of brain Tregs in TBI-aCD3 vs. TBI-Iso identified using DESeq2 analysis (two-sided Wald test, FDR-corrected P < 0.05). Due to the low number of FoxP3⁺ cells recruited to the brain, and ethical considerations, we limited the study to two biological replicates, following practices from previous studies in the field23. Despite this limitation, the consistent and robust results observed support the validity of our findings.
(a) 用于鉴定和分选脑损伤同侧半球及血液中 CD4 + FoxP3(GFP) + 细胞的设门策略。(b) 本研究中脑损伤组与假手术组血液 Treg 细胞的差异表达基因与另一项研究脑卒中后脑部 vs 血液 Treg 细胞转录组效应的重叠分析 23 。(c) 基于 DESeq2 分析(双侧 Wald 检验,FDR 校正 P<0.05)筛选的 TBI-aCD3 组与 TBI-Iso 组血液 Treg 细胞在损伤后 7 天的差异表达基因,通过 IPA 分析预测的顶级调控因子。单侧 Fisher 精确检验。*P<0.05,**P<0.01,***P<0.001。选择 FDR 校正 P<0.05 的结果。(d) 基于 DESeq2 分析(双侧 Wald 检验,FDR 校正 P<0.05)鉴定的 TBI-aCD3 组与 TBI-Iso 组脑部 Treg 细胞差异表达基因,通过 IPA 分析预测的上游调控因子。由于募集至脑部的 FoxP3⁺细胞数量较少及伦理考量,本研究遵循该领域既往研究惯例 23 仅采用两个生物学重复。尽管存在此局限,观察到的稳定可靠结果仍支持本研究发现的有效性。
We first examined the effects of TBI and nasal aCD3 treatment on blood FoxP3 Treg cells (Fig. 2g, Supplementary Table 1). We found that FoxP3 Treg cells isolated from nasally treated aCD3 TBI mice had a unique transcriptomic signature with upregulation of several genes involved in Treg cell proliferation and homeostasis (Cd47 (ref. 39), Ndfip1 (ref. 40), Cd2 (ref. 41)) and Treg cell function (Lef1 (ref. 42), Lgals1 (ref. 43) and Runx1 (ref. 44)). Ingenuity pathway analysis (IPA) revealed IL-10 as a top activated upstream regulator in blood aCD3-induced FoxP3 Treg cells compared with TBI-iso FoxP3 Treg cells along with other transcription factors relevant for Treg cell development and function (Foxo3 and Foxo4)45 (Extended Data Fig. 5c). Notably, both Foxo4 and Stat3 have been reported to regulate IL-10 transcription in CD4+ Treg cells46.
我们首先检测了创伤性脑损伤(TBI)和鼻腔 aCD3 治疗对血液 FoxP3 T 细胞的影响(图 1,补充表 2)。研究发现,从鼻腔给予 aCD3 治疗的 TBI 小鼠中分离的 FoxP3 T 细胞具有独特的转录组特征,表现为多个参与 T 细胞增殖与稳态(Cd47(参考文献 5)、Ndfip1(参考文献 6)、Cd2(参考文献 7))以及 T 细胞功能(Lef1(参考文献 9)、Lgals1(参考文献 10)和 Runx1(参考文献 11))的基因表达上调。Ingenuity 通路分析(IPA)显示,与 TBI-iso FoxP3 T 细胞相比,血液 aCD3 诱导的 FoxP3 T 细胞中 IL-10 是最活跃的上游调节因子,同时还包括其他与 T 细胞发育和功能相关的转录因子(Foxo3 和 Foxo4)(扩展数据图 16)。值得注意的是,已有报道表明 Foxo4 和 Stat3 均可调控 CD4 T 细胞中 IL-10 的转录。
We then examined the effects of TBI and nasal aCD3 treatment on brain-infiltrating Treg cells (Fig. 2h and Supplementary Table 1). We found both TBI groups (treated and untreated) had upregulated genes enriched in immune regulation (Spp1, Lgals3, Arg1, Ccl3, Ccl8, Hmox1, Ctsl, Lgals1, and Il1rn) (Fig. 2h). In addition, nasal aCD3-induced Treg cells had further increased expression of genes involved in immunomodulation (Lrp1, Tyrobp, Cxcl10, and Itgam), regulation of phagocytosis (Rab31 and Rab7), neurotrophic factors (Igf1 and Psap), lipid homeostasis (Abca1 and Lpl) and other genes required for Treg cell immunosuppressive function (Dab2 (ref. 47), Plau48 and Lgmn49). Gene set enrichment analysis (GSEA) and IPA revealed enrichment of biological pathways involved in migration, regulation of immune response, phagocytosis, neurogenesis, homeostasis and secretory functions in brain TBI-aCD3-FoxP3 Treg cells compared with brain sham-iso controls (Fig. 2i) and TBI-iso-FoxP3 Treg cells (Fig. 2j). Similar to blood FoxP3+ Treg cells, IPA identified IL-10 and Stat3 among the most activated upstream regulators of Treg cells in the brain of TBI mice treated with nasal aCD3 (Fig. 2k and Extended Data Fig. 5d). The IL-10–Stat3 axis has been reported as playing a role in the immune tolerance conferred by Treg cells50; consistent with this, flow cytometry analysis of brain Treg cells showed upregulation of IL-10 expression in aCD3-treated animals compared with TBI-iso controls (Fig. 2l). Taken together, these findings demonstrate that peripheral and central Treg cells possess unique immunomodulatory profiles associated with the amelioration of TBI via nasal aCD3 treatment.
随后我们检测了 TBI 和鼻腔 aCD3 治疗对脑浸润 T reg 细胞的影响(图 2h 和附表 1 )。发现两个 TBI 组(治疗组与未治疗组)均出现免疫调节相关基因(Spp1、Lgals3、Arg1、Ccl3、Ccl8、Hmox1、Ctsl、Lgals1 和 Il1rn)上调(图 2h )。此外,鼻腔 aCD3 诱导的 T reg 细胞还进一步增加了以下基因表达:免疫调节相关基因(Lrp1、Tyrobp、Cxcl10 和 Itgam)、吞噬作用调控基因(Rab31 和 Rab7)、神经营养因子(Igf1 和 Psap)、脂质稳态基因(Abca1 和 Lpl)以及 T reg 细胞免疫抑制功能所需的其他基因(Dab2(参考文献 47 )、Plau 48 和 Lgmn 49 )。基因集富集分析(GSEA)和 IPA 显示,与假手术对照组(图 2i )及 TBI-iso-FoxP3 T reg 细胞组(图 2j )相比,TBI-aCD3-FoxP3 T reg 细胞中涉及迁移、免疫应答调控、吞噬作用、神经发生、稳态和分泌功能的生物通路显著富集。 与血液中的 FoxP3 + T reg 细胞类似,IPA 分析发现 IL-10 和 Stat3 是鼻腔 aCD3 治疗的 TBI 小鼠脑内 T reg 细胞最活跃的上游调节因子(图 2k 及扩展数据图 5d )。已有报道表明 IL-10-Stat3 轴在 T reg 细胞介导的免疫耐受中发挥作用 50 ;与此一致,脑部 T reg 细胞的流式细胞分析显示,与 TBI-iso 对照组相比,aCD3 治疗组动物的 IL-10 表达上调(图 2l )。综上所述,这些发现表明外周和中枢 T reg 细胞具有独特的免疫调节特征,这些特征与鼻腔 aCD3 治疗改善 TBI 相关。
Nasal aCD3 mAb modulates the microglial inflammation after TBI
鼻腔给予 aCD3 单抗调控 TBI 后小胶质细胞炎症反应
Microglia play a critical role in TBI pathogenesis and their adherent activation contributes to long-term functional deficits after TBI20. Nasal aCD3 treatment increased the migration of FoxP3+ Treg cells to the meninges and CCI lesion site (Fig. 2e), where they were found to be in close contact with microglial dendrites after injury (Fig. 3a). Thus, to further elucidate the effects of TBI and immediate nasal aCD3 treatment on the microglial inflammatory profile post-injury, we performed RNA-seq analysis on sorted microglial single-cell suspensions from the ipsilateral hemisphere of the mouse brains, using the microglia-specific 4D4+ antibody51 (Extended Data Fig. 6a) at 7 and 30 d post-CCI (Fig. 3b and Supplementary Table 2). In analyzing the highest expressed genes in the sham-iso, TBI-iso and TBI-aCD3 microglial groups, we found that multiple microglial genes, including Cx3cr1,
Hexb and Tmem119, were among the highest expressed (Extended Data Fig. 6b). A heatmap of the microglial gene signature demonstrated that the TBI-iso and TBI-aCD3 groups had overall similar transcriptomic profiles at 7 d, yet with demonstration of early upregulation of anti-inflammatory genes such as Cx3cr1, CD33, Hspa1a, and Hspa1b, whereas there was a clear modulation of the microglial transcriptomic signature in TBI-aCD3 group toward the sham-iso phenotype at 30 d post-injury (Fig. 3c and Supplementary Table 2), including the downregulation of microglial proinflammatory genes (Il6, Il18, Cd36, Ifitm3, and Lgals1) and upregulation of key homeostatic genes (Tgfbr2, Adgrg1, Mertk, Rhob, Atp8a2, Abcc3, Fscn1, Pde3b, Inpp4b, and Cmklr1).
小胶质细胞在 TBI 发病机制中起关键作用,其持续性激活会导致 TBI 后长期功能缺损 20 。鼻腔 aCD3 治疗增加了 FoxP3 + T reg 细胞向脑膜和 CCI 损伤部位的迁移(图 2e ),这些细胞被发现与损伤后的小胶质细胞树突密切接触(图 3a )。为进一步阐明 TBI 及鼻腔 aCD3 即刻治疗对损伤后小胶质细胞炎症表型的影响,我们使用小胶质细胞特异性抗体 4D4 + 51 (扩展数据图 6a ),对 CCI 后 7 天和 30 天小鼠大脑同侧半球分选的小胶质细胞单细胞悬液进行 RNA-seq 分析(图 3b 及补充表 2 )。在分析假手术组、TBI 对照组和 TBI-aCD3 治疗组小胶质细胞中高表达基因时,发现包括 Cx3cr1、Hexb 和 Tmem119 在内的多个小胶质细胞基因表达量最高(扩展数据图 6b )。 小胶质细胞基因特征的热图分析显示,TBI-iso 组和 TBI-aCD3 组在损伤后 7 天具有总体相似的转录组特征,但已表现出抗炎基因(如 Cx3cr1、CD33、Hspa1a 和 Hspa1b)的早期上调。而到损伤后 30 天时,TBI-aCD3 组的小胶质细胞转录组特征明显向假手术组表型转变(图 3c 和补充表 2 ),表现为小胶质细胞促炎基因(Il6、Il18、Cd36、Ifitm3 和 Lgals1)的下调,以及关键稳态基因(Tgfbr2、Adgrg1、Mertk、Rhob、Atp8a2、Abcc3、Fscn1、Pde3b、Inpp4b 和 Cmklr1)的上调。
Fig. 3. Nasal aCD3 modulates the microglial inflammatory response after TBI.
图 3. 鼻腔给予 aCD3 调节 TBI 后小胶质细胞的炎症反应
a, Immunofluorescence of ipsilateral brain lesion (7 d post-TBI) from FoxP3-GFP mice for DAPI (blue), CD3 (pink), FoxP3 (green) and Iba-1 (red) showing FoxP3 Treg cells in close proximity to Iba-1. Scale bars, 100 μm and 50 μm for the enlarged image. b, Experimental timeline schematic for microglial bulk RNA-seq at 7 and 30 d after TBI and treatment (created with BioRender.com). c, Heatmap of DEGs from microglia at 7 and 30 d post-TBI identified using DESeq2 analysis (two-sided LRT, n = 4 mice per group, FDR-corrected P < 0.05). d, Microglial core sensome genes at 7 and 30 d in TBI-aCD3 versus TBI-iso. Genes are colored by their function53,107,108. Emboldened DEGs have an asterisk: FDR-corrected P < 0.05; *P < 0.05 (DESeq2 analysis, two-sided Wald’s test, n = 4 mice per group). e, GSEA of GOBP at 7 and 30 d post-TBI based on the following pairwise comparisons: TBI-iso versus sham-iso and TBI-aCD3 versus sham-iso; the asterisk indicates enriched terms (q-value < 0.05). NES, normalized enrichment score. f, Heatmap of genes involved in inflammatory response from microglia at 30 d post-TBI. Genes identified with an FDR-corrected P < 0.05 using DESeq2 analysis are indicated by an asterisk (two-sided LRT, n = 4 mice per group). Genes were identified from the GOBP term inflammatory response as well as microglial inflammatory genes89. g, Heatmap of DAM and MGnD genes at 30 d post-TBI. Genes identified with an FDR-corrected P < 0.05 using DESeq2 analysis are indicated by an asterisk (two-sided LRT, n = 4 mice per group). Genes were identified based on the previous work of our group and others51,61. h, RT–qPCR of microglia sorted from the ipsilateral hemisphere at 7 and 30 d post-TBI. Expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and presented relative to that of sham-iso animals (sham-iso n = 4, TBI-iso n = 5, TBI-aCD3 n = 5), analyzed by one-way ANOVA with Tukey’s multiple comparisons. Data are shown as mean ± s.e.m. All data are biological replicates and represent two independent experiments.
a,FoxP3-GFP 小鼠创伤性脑损伤后 7 天同侧脑损伤区域的免疫荧光染色:DAPI(蓝色)、CD3(粉红色)、FoxP3(绿色)和 Iba-1(红色)显示 FoxP3 T 细胞与 Iba-1 紧密相邻。比例尺:主图 100 微米,放大图 50 微米。b,创伤性脑损伤后 7 天和 30 天小胶质细胞批量 RNA 测序及治疗实验时间轴示意图(使用 BioRender.com 制作)。c,采用 DESeq2 分析鉴定的创伤性脑损伤后 7 天和 30 天小胶质细胞差异表达基因热图(双侧似然比检验,每组 n=4 只小鼠,FDR 校正 P<0.05)。d,TBI-aCD3 组与 TBI-iso 组在 7 天和 30 天时小胶质细胞核心感应组基因表达。基因按功能 53,107,108 着色。加粗的差异表达基因标有星号:FDR 校正 P<0.05; * P<0.05(DESeq2 分析,双侧 Wald 检验,每组 n=4 只小鼠)。e,基于以下组间比较的创伤性脑损伤后 7 天和 30 天 GOBP 基因集富集分析:TBI-iso 组对比假手术-iso 组、TBI-aCD3 组对比假手术-iso 组;星号标注富集条目(q 值<0.05)。NES,标准化富集分数。f,创伤性脑损伤后 30 天小胶质细胞炎症反应相关基因热图。 经 DESeq2 分析(双侧 LRT,每组 n=4 只小鼠)以 FDR 校正 P 值<0.05 筛选的基因用星号标注。这些基因来源于 GOBP 术语"炎症反应"条目及小胶质细胞炎症相关基因 89 。图 g 显示创伤性脑损伤(TBI)后 30 天的 DAM 与 MGnD 基因热图。经 DESeq2 分析(双侧 LRT,每组 n=4 只小鼠)以 FDR 校正 P 值<0.05 筛选的基因用星号标注,基因筛选基于本课题组及他人既往研究 51,61 。图 h 为 TBI 后 7 天和 30 天同侧半球分选小胶质细胞的 RT-qPCR 结果,表达量以甘油醛-3-磷酸脱氢酶(GAPDH)为内参,数据相对于假手术对照组(假手术组 n=4,TBI 对照组 n=5,TBI-aCD3 组 n=5)呈现,采用单因素方差分析结合 Tukey 多重比较。数据以均值±标准误表示,所有数据均为生物学重复,代表两次独立实验。
Extended Data Fig. 6. Nasal anti-CD3 modulates chronic microglial response after TBI for different treatment regimens and TBI severities.
扩展数据图 6. 鼻腔给予抗 CD3 单抗通过不同治疗方案调节 TBI 后小胶质细胞慢性反应(适用于不同创伤严重程度)
(a) Gating strategy for microglia. (b) Relative expression of cell types in Sham-Iso microglia (n = 3). (c) RT- qPCR of ipsilateral hemisphere 7 and 30 days post-TBI (immediate treatment males). Expression was normalized to GAPDH and presented relative to Sham-Iso. Data shown as mean ± SEM, (Sham-Iso n = 6, TBI-Iso n = 8, TBI-aCD3 n = 8) and analyzed by one-way ANOVA with Tukey’s multiple comparisons. (d) Heatmap signature of DEGs 30 days post-TBI (early treatment males) identified using DESeq2 analysis (two-sided likelihood ratio test, n = 5 mice/group, FDR-corrected P < 0.05). (e) Heatmap of genes in inflammatory response and genes of disease-associated microglia (DAM) and neurodegenerative microglia (MGnD) 30 days post-TBI (early treatment males). Genes identified with an FDR-corrected p-value < 0.05 using DESeq2 analysis are bolded (two-sided likelihood ratio test, n = 5 mice/group). (f) GSEA analysis of GO Biological Processes (BP) comparing TBI-aCD3 vs. TBI-Iso groups 30 days post-TBI (early treatment male mice). NES, normalized enrichment score. (g) RT- qPCR of ipsilateral hemisphere 30 days post-TBI (early treatment males). Expression normalized to GAPDH and presented relative to Sham-Iso. Data shown as mean ± SEM, (Sham-Iso n = 5, TBI-Iso n = 6, TBI-aCD3 n = 6) and analyzed by one-way ANOVA with Tukey’s multiple comparisons. (h) Venn diagram of DEGs in comparisons with Sham-Iso microglia group as baseline 30 days post-severe TBI (immediate treatment female mice): TBI-Iso, and TBI-aCD3. (i) Heatmap genes involved in inflammatory response and (DAM) and (MGnD) at 30 days following severe TBI (immediate treatment females). Genes identified with FDR-corrected p-value < 0.05 using DESeq2 analysis are bolded (two-sided likelihood ratio test, n = 5 mice/group). (j) RT- qPCR of ipsilateral hemisphere 30 days post-severe TBI (immediate treatment female). Expression was normalized to GAPDH and presented relative to Sham-Iso. Data shown as mean ± SEM, (Sham-Iso n = 5, TBI-Iso n = 6, TBI-aCD3 n = 6) and analyzed by one-way ANOVA with Tukey’s multiple comparisons. All data are biological replicates and are representative from two independent experiments. n.s. = non-significant.
(a) 小胶质细胞的分选策略。(b) Sham-Iso 组小胶质细胞中各细胞类型的相对表达量(n = 3)。(c) TBI 后 7 天和 30 天损伤同侧大脑半球的 RT-qPCR 结果(即刻治疗雄性组)。表达量以 GAPDH 为内参,相对于 Sham-Iso 组标准化。数据显示为均值±SEM(Sham-Iso n = 6,TBI-Iso n = 8,TBI-aCD3 n = 8),采用单因素方差分析及 Tukey 多重比较检验。(d) TBI 后 30 天差异表达基因(DEGs)的热图特征(早期治疗雄性组),通过 DESeq2 分析鉴定(双侧似然比检验,n = 5 只/组,FDR 校正 P < 0.05)。(e) TBI 后 30 天炎症反应相关基因及疾病相关小胶质细胞(DAM)与神经退行性小胶质细胞(MGnD)基因热图(早期治疗雄性组)。通过 DESeq2 分析鉴定 FDR 校正 p 值<0.05 的基因以加粗显示(双侧似然比检验,n = 5 只/组)。(f) TBI 后 30 天 TBI-aCD3 组与 TBI-Iso 组的 GO 生物过程(BP)GSEA 分析(早期治疗雄性小鼠组)。NES,标准化富集分数。(g) TBI 后 30 天损伤同侧大脑半球的 RT-qPCR 结果(早期治疗雄性组)。 以 GAPDH 为内参进行标准化表达,数据表示为相对于 Sham-Iso 组的相对值(Sham-Iso 组 n=5,TBI-Iso 组 n=6,TBI-aCD3 组 n=6),采用单因素方差分析及 Tukey 多重比较。(h)以 Sham-Iso 小胶质细胞组为基线(严重 TBI 后 30 天,雌性小鼠即刻治疗组),通过维恩图展示 TBI-Iso 组与 TBI-aCD3 组的差异表达基因。(i)热图显示严重 TBI 后 30 天(雌性即刻治疗组)炎症反应相关基因及(DAM)与(MGnD)相关基因。经 DESeq2 分析(双侧似然比检验,每组 n=5),FDR 校正 p 值<0.05 的基因以加粗标示。(j)严重 TBI 后 30 天(雌性即刻治疗组)损伤同侧半球的 RT-qPCR 结果。以 GAPDH 为内参进行标准化表达,数据表示为相对于 Sham-Iso 组的相对值(Sham-Iso 组 n=5,TBI-Iso 组 n=6,TBI-aCD3 组 n=6),采用单因素方差分析及 Tukey 多重比较。所有数据均为生物学重复,结果来自两次独立实验。n.s.表示无统计学意义。
Microglia possess a unique transcriptomic signature that enables them to perform sensing, homeostatic and housekeeping functions, which vary according to the brain’s physiological or pathological state52. To determine the effects of TBI and nasal anti-CD3 on these essential microglial functions, we examined the microglial sensome dataset for genes and pathways involved in each of these functions53. At 7 d post-TBI, we found that microglia from the nasal aCD3-treated group, compared with TBI-iso, were associated with increased expression of homeostatic and sensing genes involved in pattern recognition receptors (Tlr1), Fc receptors (Cmtm7), Siglec receptors (Cd33), cell–cell interaction (Cd84 and Lag3) and chemokine receptors (Cx3cr1) (Fig. 3d). Cd33 and Lag3 were among the most significantly differentially expressed genes (DEGs) in the TBI aCD3-treated group compared with TBI-iso control at 7 d post-injury. CD33 activity has been implicated in processes including microglial endogenous ligand receptors and sensors, adhesion processing of immune cells and inhibition of cytokines release by monocytes54,55. Lymphocyte activation gene-3 (Lag3) regulates T cell expansion and limits the duration and intensity of the immune response56. Moreover, the TBI aCD3-treated group exhibited downregulation of Cd14, a key regulator of microglial proinflammatory responses to injury57. At 30 d post-injury, we found that nasal aCD3 treatment was associated with increased expression of several transforming growth factor β (TGF-β)-signaling genes, including Smad3, Tgfbr1 and Tgfbr2 compared with TBI-iso control (Fig. 3c,d and Supplementary Table 2)58. TGF-β is required for maintaining the microglial homeostatic state59 and modulating microglia-mediated inflammation after acute brain injury60. Nasal aCD3 treatment was also associated with decreased expression of sensing genes involved in cytokine receptor (Tnfrsf17), FC receptor (Fcgr1, Fcgr4, Fcgr3, and Fcer1g) and pattern recognition receptors (Cd74, Tlr6, Upk1b, and Selplg) compared with TBI-iso control (Fig. 3d).
小胶质细胞具有独特的转录组特征,使其能够执行感知、稳态维持和管家功能,这些功能会随大脑生理或病理状态的变化而改变 52 。为探究创伤性脑损伤(TBI)和鼻腔抗 CD3 单抗对这些关键小胶质细胞功能的影响,我们检测了参与各项功能的微胶质感知组基因及通路 53 。在 TBI 后第 7 天,与 TBI-iso 组相比,鼻腔 aCD3 治疗组的小胶质细胞显示出参与模式识别受体(Tlr1)、Fc 受体(Cmtm7)、Siglec 受体(Cd33)、细胞间相互作用(Cd84 和 Lag3)以及趋化因子受体(Cx3cr1)的稳态与感知基因表达上调(图 3d )。其中 Cd33 和 Lag3 是 TBI 后 7 天 aCD3 治疗组相较于 TBI-iso 对照组差异最显著的基因(DEGs)。CD33 活性已被证实参与微胶质细胞内源性配体受体与传感器的调控、免疫细胞粘附处理以及单核细胞细胞因子释放抑制等过程 54,55 。 淋巴细胞激活基因-3(Lag3)可调节 T 细胞增殖并限制免疫反应的持续时间和强度 56 。此外,创伤性脑损伤 aCD3 治疗组表现出 Cd14(小胶质细胞对损伤促炎反应的关键调节因子)的下调 57 。在损伤后 30 天,我们发现与 TBI-iso 对照组相比,鼻腔 aCD3 治疗与多个转化生长因子β(TGF-β)信号通路基因(包括 Smad3、Tgfbr1 和 Tgfbr2)表达增加相关(图 3c,d 和补充表 2 ) 58 。TGF-β是维持小胶质细胞稳态 59 以及调节急性脑损伤后小胶质细胞介导的炎症反应所必需的 60 。与 TBI-iso 对照组相比,鼻腔 aCD3 治疗还导致细胞因子受体(Tnfrsf17)、FC 受体(Fcgr1、Fcgr4、Fcgr3 和 Fcer1g)及模式识别受体(Cd74、Tlr6、Upk1b 和 Selplg)相关感知基因表达降低(图 3d )。
We then performed GSEA comparing the TBI groups with sham-iso controls at 7 and 30 d post-injury. At 7 d post-injury, we observed an upregulation in pathways involved in oxidative stress and neuron apoptosis in the TBI-iso group compared with the sham-iso group. The TBI-aCD3-treated animals had less upregulation of genes in these pathways and more upregulation in microglial pathways involved in the regulation of anti-inflammatory IL-10 production, phagocytosis and tolerance induction at 7 d post-injury (Fig. 3e). At 30 d post-TBI, we found enrichment of pathways involved in proinflammatory mechanisms (IL-6, IL-1 and tumor necrosis factor production), adaptive immune response, T cell cytotoxicity and cell killing pathways in the TBI-iso control compared with the sham-iso group. However, TBI-aCD3-treated animals had less upregulation of genes in these proinflammatory pathways and more upregulation in biological pathways involved in regulation of phagocytosis and cytokine production (Fig. 3e).
随后我们通过 GSEA 分析比较了创伤性脑损伤组与假手术对照组在伤后 7 天和 30 天的基因表达差异。伤后 7 天时,与假手术组相比,TBI-iso 组显示出氧化应激和神经元凋亡相关通路上调。而经 TBI-aCD3 治疗的动物在这些通路中的基因上调程度较轻,同时在调控抗炎因子 IL-10 生成、小胶质细胞吞噬功能及免疫耐受诱导等通路上调更为显著(图 3e )。至创伤后 30 天,与假手术组相比,TBI-iso 对照组中促炎机制(IL-6、IL-1 及肿瘤坏死因子生成)、适应性免疫应答、T 细胞毒性及细胞杀伤相关通路呈现富集。但 TBI-aCD3 治疗组动物在这些促炎通路中的基因上调减弱,而在调控吞噬作用与细胞因子生成的生物学通路上调更为明显(图 3e )。
Previous studies have suggested an association between microglia-mediated chronic inflammation after TBI and subsequent chronic neurodegeneration20,52. To investigate this relationship, we analyzed the expression levels of inflammatory response genes and genes characteristic of disease-associated microglia (DAMs)61 and neurodegenerative microglia (MGnDs)51 in all three groups at 30 d post-injury. We found that the TBI-iso microglia had a unique proinflammatory response (Fig. 3f) and a DAM or MGnD signature (Fig. 3g), because we found increased expression of key proinflammatory (Casp1, Nfkbia, C5ar1, Lyz1, Ifitm3, Il6, Lyz2, Cd86, and Irgm1), DAM-1 and -2 genes (B2m, Cstb, Cd52, Cd9, Cst7, Fth1, Ccl6, and Tyrobp) and MGnD microglial genes (Cybb, Gpnmb, Lgals3, and Clec7a)51,61. Importantly, several of these genes were downregulated in nasal aCD3-treated mice, approaching expression levels observed in the sham group (Fig. 3f-g).
先前研究表明,创伤性脑损伤(TBI)后小胶质细胞介导的慢性炎症与后续慢性神经退行性病变存在关联 20,52 。为探究这一关系,我们在损伤后 30 天检测了三组实验对象的炎症反应基因表达水平,以及疾病相关小胶质细胞(DAMs) 61 和神经退行性小胶质细胞(MGnDs) 51 的特征基因。研究发现 TBI-iso 组小胶质细胞呈现独特的促炎反应(图 3f )及 DAM/MGnD 特征(图 3g ),表现为关键促炎基因(Casp1、Nfkbia、C5ar1、Lyz1、Ifitm3、Il6、Lyz2、Cd86 和 Irgm1)、DAM-1/-2 基因(B2m、Cstb、Cd52、Cd9、Cst7、Fth1、Ccl6 和 Tyrobp)及 MGnD 小胶质细胞基因(Cybb、Gpnmb、Lgals3 和 Clec7a)表达上调 51,61 。值得注意的是,经鼻腔 aCD3 单抗治疗的小鼠中,多个基因表达下调至接近假手术组水平(图 3f-g )。
Consistent with the microglial transcriptomic data, quantitative PCR with reverse transcription (RT–qPCR) from ipsilateral hemisphere sorted microglia (Fig. 3h) and brain tissue (Extended Data Fig. 6c) revealed that, compared with the TBI-iso control, nasal aCD3 treatment increased the expression of the anti-inflammatory cytokine Il10 in both microglia and brain tissue at 7 d post-injury. Moreover, treatment reduced several proinflammatory markers in microglia (Clec7a, Tlr2, Il1b, Tnf, Cd86, and Il18) and brain tissue (Il6, Tnf, Ifng, Il17a, and Ccl5) at 30 d post-injury. Of note, mice treated with nasal aCD3 showed upregulation of brain-derived neurotrophic factor (Bdnf), a neurotrophin that has a critical role in neuronal survival and is involved in synaptic plasticity, learning and memory62, compared with the TBI-iso control, at 1 month post-injury (Extended Data Fig. 6c).
与小胶质细胞转录组数据一致,通过同侧半球分选小胶质细胞(图 3h )及脑组织(扩展数据图 6c )的反转录定量 PCR(RT-qPCR)显示:与 TBI-iso 对照组相比,鼻腔给予 aCD3 治疗在损伤后 7 天同时提升了小胶质细胞和脑组织中抗炎细胞因子 Il10 的表达水平。此外,该治疗在损伤后 30 天显著降低了小胶质细胞(Clec7a、Tlr2、Il1b、Tnf、Cd86 和 Il18)与脑组织(Il6、Tnf、Ifng、Il17a 和 Ccl5)中多种促炎标志物的表达。值得注意的是,损伤后 1 个月时(扩展数据图 6c ),鼻腔 aCD3 治疗组小鼠相比 TBI-iso 对照组显示出脑源性神经营养因子(Bdnf)的上调——这种神经营养素对神经元存活至关重要,并参与突触可塑性、学习与记忆过程 62 。
To further investigate whether delaying the therapeutic window of nasal anti-CD3 to 3 d post-injury would still modulate the microglial transcriptomic profile at 30 d post-injury, we performed bulk RNA-seq on isolated microglia from mice treated with nasal aCD3 or isotype control from day 3 to day 30 (early treatment) post-injury (Supplementary Table 2). We found that the early treatment paradigm also modulated the microglial transcriptomic signature in the TBI-aCD3 group toward the sham-iso phenotype (Extended Data Fig. 6d) and was associated with decreased expression of several proinflammatory and DAM or MGnD genes (Lpl, Lyz1, Nfkbia, Irgm1, Lyz2 and Apoe) (Extended Data Fig. 6e). Consistent with this, GSEA analysis revealed that the early nasal aCD3 treatment paradigm was also associated with downregulation in several inflammatory response and immune response-related pathways compared with TBI-iso (Extended Data Fig. 6f). In line with these results, RT–qPCR of ipsilateral brain tissue demonstrated a reduction in several proinflammatory genes including Il6, Il18 and Tnf in TBI-aCD3 compared with TBI-iso controls (Extended Data Fig. 6g).
为进一步探究伤后 3 天延迟给予鼻用抗 CD3 单抗治疗是否仍能调控伤后 30 天小胶质细胞的转录组特征,我们对伤后第 3 至 30 天接受鼻用 aCD3 或同型对照(早期治疗组)的小鼠分离小胶质细胞进行批量 RNA 测序(补充表 2 )。研究发现早期治疗模式同样使 TBI-aCD3 组的小胶质细胞转录特征向假手术-同型对照组表型转变(扩展数据图 6d ),并伴随多种促炎基因及 DAM/MGnD 相关基因(Lpl、Lyz1、Nfkbia、Irgm1、Lyz2 和 Apoe)表达下调(扩展数据图 6e )。与此一致的是,GSEA 分析显示相较于 TBI-同型对照组,早期鼻用 aCD3 治疗方案还导致多种炎症反应和免疫反应相关通路下调(扩展数据图 6f )。与这些结果相印证,同侧脑组织的 RT-qPCR 检测显示,相比 TBI-同型对照组,TBI-aCD3 组中包括 Il6、Il18 和 Tnf 在内的多个促炎基因表达降低(扩展数据图 6g )。
To assess the effect of nasal aCD3 treatment on microglia inflammation in female mice after severe TBI, we performed bulk RNA-seq on sorted microglia at 30 d post-injury (Supplementary Table 2). We found a similar therapeutic effect in female mice with the TBI-aCD3 microglial transcriptomic profile reverting to that of sham mice, associated with a reduced inflammatory and DAM or MGnD profile relative to TBI-iso mice controls (Extended Data Fig. 6h,i). RT–qPCR of ipsilateral brain tissue showed consistent findings (Extended Data Fig. 6j). Taken together, these findings demonstrate that nasal aCD3 shifts microglia from a pathogenic, disease-associated phenotype to a beneficial, homeostatic phenotype.
为评估鼻腔 aCD3 治疗对雌性小鼠严重 TBI 后小胶质细胞炎症的影响,我们在损伤后 30 天对分选的小胶质细胞进行了批量 RNA 测序(补充表 2 )。研究发现雌性小鼠具有相似治疗效果,TBI-aCD3 组小胶质细胞转录组特征恢复至假手术组水平,相较于 TBI-iso 对照组表现出炎症反应及 DAM/MGnD 特征减弱(扩展数据图 6h,i )。同侧脑组织 RT-qPCR 结果与此一致(扩展数据图 6j )。综上表明,鼻腔 aCD3 能使小胶质细胞从致病性、疾病相关表型转变为有益的内稳态表型。
Nasal aCD3 increases microglia phagocytosis in an IL-10-dependent manner
鼻腔给予 aCD3 通过 IL-10 依赖性途径增强小胶质细胞吞噬功能
Acute brain injury leads to neuronal cell death and the release of substantial amounts of myelin and cell debris, which subsequently trigger a persistent and intense inflammatory response that may impede neurological recovery. Microglia and macrophages play an important role in debris removal and modulating the immune response post-injury20. However, uncontrolled phagocytosis may lead to progressive brain damage and worsening cognitive and memory impairments63. We found that TBI-aCD3-treated animals had increased upregulation in biological pathways involved in regulation of phagocytosis (Fig. 3e) and a distinct upregulated phagocytic signature at 7 d post-injury (Fig. 4a), with increased expression of several key microglial phagocytosis regulators at 30 d post-TBI, including Mertk64, Sirpa65 and Tlr4 (ref. 66), compared with TBI-iso microglia (Supplementary Table 2). To functionally assess the phagocytic capacity of microglia after TBI (with and without nasal aCD3 treatment), we performed an in vivo experiment in which the TBI-induced lesion was injected with either labeled apoptotic neurons or phosphate-buffered saline (PBS) on day 6 post-injury for 16 h and on day 7 post-injury for 4 h post-injection experiments (Fig. 4b). In line with our microglial transcriptomic data, aCD3-treated animals had higher microglial phagocytic capacity to uptake apoptotic neurons at both 4 and 16 h post-injection compared with the TBI-iso group (Fig. 4c,d and Extended Data Fig. 7a,b). To elucidate the mechanism by which nasal aCD3 enhanced microglial phagocytic capacity after TBI, we performed bulk RNA-seq on phagocytic and nonphagocytic microglia isolated at 4 and 16 h post-injection from TBI-iso and TBI-aCD3 groups at 7 d post-TBI (Supplementary Table 3). At 4 h post-injection, we observed a distinct microglial transcriptomic signature for phagocytic TBI-aCD3 microglia, characterized by the upregulation of several key phagocytosis genes (Fig. 4e). Compared with nonphagocytic TBI-iso microglia, phagocytic TBI-aCD3 microglia were associated with increased expression of genes involved in the recognition and engulfment (eat me and find me signals) of apoptotic cells and debris (Mertk67, Mrc1 (ref. 68), Abca1 (ref. 67), Lrp1 (ref. 67), and Stab1 (ref. 69)), digestion and degradation of engulfed material including lysosomal machinery (Rab27a70, Smcr8 (ref. 71), Clec16a72, and Vps8 (ref. 73)), lipid metabolism (Apoc1 (ref. 74), Olr1 (ref. 75) and Ldlr76), cytoskeleton dynamics pathways (Myo1e77) and regulation of microglial phagocytosis (Tspo78, Qk79 and Pik3cg80) (Fig. 4e,f and Supplementary Table 3). Consistent with these findings, GSEA analysis of phagocytic TBI-aCD3 microglia compared with nonphagocytic TBI-iso microglia showed enrichment for pathways involved in phagocytosis, along with other pathways pertinent for the phagocytic process such as endocytosis, pattern recognition and cell migration, all of which were not upregulated in the phagocytic TBI-iso microglia (Fig. 4g). In addition, compared with phagocytic TBI-iso microglia, phagocytic TBI-aCD3 microglia had increased upregulation of antigen presentation and IL-10 pathways (Fig. 4h). IPA analysis revealed IL-10 as a top regulator and signaling pathway in aCD3-treated phagocytic microglia post-TBI (Fig. 4h,i). We found a similar pattern of increased expression of phagocytosis machinery-related genes and pathways at 16 h post-injection (Extended Data Fig. 7c) and also observed that phagocytic TBI-aCD3 microglia had a more homeostatic and a less inflammatory (disease-associated) profile compared with phagocytic TBI-iso microglia (Extended Data Fig. 7d,e and Supplementary Table 3).
急性脑损伤会导致神经元细胞死亡并释放大量髓鞘和细胞碎片,这些物质随后会引发持续而强烈的炎症反应,可能阻碍神经功能恢复。小胶质细胞和巨噬细胞在损伤后清除碎片及调节免疫反应中起重要作用 20 。然而不受控制的吞噬作用可能导致进行性脑损伤及认知记忆功能恶化 63 。我们发现 TBI-aCD3 治疗组动物在吞噬作用调控相关生物通路上调增加(图 3e ),并在损伤后 7 天呈现显著上调的吞噬特征(图 4a ),与 TBI-iso 组小胶质细胞相比,损伤后 30 天多个关键小胶质细胞吞噬调控因子(包括 Mertk 64 、Sirpa 65 和 Tlr4(参考文献 66 ))表达增加(补充表 2 )。 为功能性评估创伤性脑损伤(TBI)后小胶质细胞的吞噬能力(含/不含鼻腔 aCD3 治疗),我们进行了体内实验:在损伤后第 6 天向 TBI 病灶注射标记的凋亡神经元或磷酸盐缓冲液(PBS)持续 16 小时,并于损伤后第 7 天进行注射后 4 小时实验(图 4b )。与小胶质细胞转录组数据一致,与 TBI-iso 组相比,aCD3 治疗组动物在注射后 4 小时和 16 小时均表现出更强的凋亡神经元摄取能力(图 4c,d 及扩展数据图 7a,b )。为阐明鼻腔 aCD3 增强 TBI 后小胶质细胞吞噬能力的机制,我们对 TBI 后 7 天从 TBI-iso 组和 TBI-aCD3 组分离的吞噬性与非吞噬性小胶质细胞进行了批量 RNA 测序(补充表 3 )。注射后 4 小时,我们观察到吞噬性 TBI-aCD3 小胶质细胞具有独特的转录组特征,表现为多个关键吞噬基因的上调(图 4e )。 与非吞噬型的 TBI-iso 小胶质细胞相比,吞噬型 TBI-aCD3 小胶质细胞表现出以下基因表达上调:参与凋亡细胞和碎片识别与吞噬("吃我"和"找我"信号)的基因(Mertk 67 、Mrc1(参考文献 68 )、Abca1(参考文献 67 )、Lrp1(参考文献 67 )和 Stab1(参考文献 69 ))、被吞噬物质的消化降解相关基因(包括溶酶体机制相关基因 Rab27a 70 、Smcr8(参考文献 71 )、Clec16a 72 和 Vps8(参考文献 73 ))、脂质代谢相关基因(Apoc1(参考文献 74 )、Olr1(参考文献 75 )和 Ldlr 76 )、细胞骨架动态通路相关基因(Myo1e 77 )以及小胶质细胞吞噬调控相关基因(Tspo 78 、Qk 79 和 Pik3cg 80 )(图 4e,f 和补充表 3 )。与这些发现一致的是,GSEA 分析显示吞噬型 TBI-aCD3 小胶质细胞相较于非吞噬型 TBI-iso 小胶质细胞在吞噬作用相关通路上呈现富集,同时还富集了与吞噬过程相关的其他通路如内吞作用、模式识别和细胞迁移——这些通路在吞噬型 TBI-iso 小胶质细胞中均未出现上调(图 4g )。 此外,与吞噬型 TBI-iso 小胶质细胞相比,吞噬型 TBI-aCD3 小胶质细胞的抗原呈递和 IL-10 通路上调更为显著(图 4h )。IPA 分析显示 IL-10 是 TBI 后 aCD3 处理吞噬型小胶质细胞中的顶级调节因子和信号通路(图 4h,i )。我们在注射后 16 小时也发现了吞噬机制相关基因和通路表达增加的相似模式(扩展数据图 7c ),并观察到与吞噬型 TBI-iso 小胶质细胞相比,吞噬型 TBI-aCD3 小胶质细胞具有更稳态且炎症(疾病相关)特征更弱的表型(扩展数据图 7d,e 及补充表 3 )。
Fig. 4. Nasal aCD3 increased microglial phagocytic capacity after TBI in an IL-10-dependent manner.
图 4. 鼻内给予 aCD3 通过 IL-10 依赖性方式增强 TBI 后小胶质细胞的吞噬能力
a, Heatmap of microglial phagocytosis genes 7 d after TBI. Genes identified with FDR-corrected P < 0.05 using DESeq2 analysis are indicated by an asterisk (two-sided LRT, n = 4 mice per group). Genes were identified from the GOBP term phagocytosis and microglial phagocytosis genes51,61. b, Schematic presenting a phagocytosis functional study (created with BioRender.com). c, Immunofluorescence of lesion (7 d post-TBI) for apoptotic neurons (blue) and P2ry12 (red) showing engulfment of apoptotic neurons by P2ry12. Scale bars, 100 μm and 50 μm for the enlarged image. d, Phagocytosis experiment where mice were injected with labeled apoptotic neurons and sacrificed 4 h post-injection. The gating strategy shows phagocytic positive microglia and data are shown as box plots (min., max., IQR, median) and n = 5 mice per group were used. Data were analyzed by two-sided, unpaired Student’s t-test. e, Clustered heatmap of DEGs of aggregated samples for phagocytic (+P) and nonphagocytic (−P) microglia 7 d post-TBI and 4 h post-injection of apoptotic neurons identified using DESeq2 analysis (two-sided LRT, n = 3-4 mice per group, FDR-corrected P < 0.05). f, Bar plots with log2(fold-changes) of genes from e pertinent to microglial phagocytosis and related functions in the following comparisons: TBI-iso (+P) versus TBI-iso (−P) and TBI-aCD3 (+P) versus TBI-Iso (−P). g, GSEA analysis of GOBP 7 d post-TBI and 4 h post-injection of apoptotic neurons based on pairwise comparisons: TBI-iso (+P) versus TBI-Iso (−P), TBI-aCD3 (+P) versus TBI-Iso (−P) and TBI-aCD3 (−P) versus TBI-Iso (−P). The asterisk indicates enriched terms (q-value < 0.05). h, Selected top canonical pathways from IPA analysis of DEGs in phagocytic TBI-aCD3 microglia compared with phagocytic and nonphagocytic TBI-iso microglial groups at 7 d post-TBI and 4 h post-injection of apoptotic neurons. i, Predicted upstream regulator in TBI-aCD3 (+P) versus TBI-iso (−P). j, Phagocytosis experiment with similar design to b. Data are shown as box plots (min., max., IQR, median) and n = 5 mice per group were used. The data were analyzed by one-way ANOVA with Tukey’s multiple comparisons. All data are biological replicates and represent two independent experiments.
a. TBI 后 7 天小胶质细胞吞噬基因热图。经 DESeq2 分析确定 FDR 校正 P 值<0.05 的基因以星号标注(双侧似然比检验,每组 n=4 只小鼠)。基因选自 GOBP 术语"吞噬作用"及小胶质细胞吞噬基因集 51,61 。
b. 吞噬功能研究示意图(使用 BioRender.com 制作)。
c. TBI 后 7 天损伤区免疫荧光染色显示凋亡神经元(蓝色)与 P2ry12(红色),呈现 P2ry12+细胞对凋亡神经元的吞噬现象。比例尺:主图 100μm,放大图 50μm。
d. 吞噬实验:注射标记凋亡神经元 4 小时后处死小鼠。流式设门策略显示吞噬阳性小胶质细胞,数据以箱线图呈现(最小值、最大值、四分位距、中位数),每组 n=5 只小鼠。采用双侧非配对 t 检验进行数据分析。 e. 采用 DESeq2 分析(双侧 LRT,每组 3-4 只小鼠,FDR 校正 P<0.05)鉴定的创伤性脑损伤(TBI)后 7 天及凋亡神经元注射后 4 小时,吞噬性(+P)与非吞噬性(-P)小胶质细胞聚合样本差异表达基因(DEGs)的聚类热图。f. 柱状图显示图 e 中与小胶质细胞吞噬及相关功能相关基因的 log2(倍数变化),比较组为:TBI-iso(+P) vs TBI-iso(-P)及 TBI-aCD3(+P) vs TBI-Iso(-P)。g. 基于配对比较的基因集富集分析(GSEA):TBI-iso(+P) vs TBI-Iso(-P)、TBI-aCD3(+P) vs TBI-Iso(-P)及 TBI-aCD3(-P) vs TBI-Iso(-P)在 TBI 后 7 天及凋亡神经元注射后 4 小时的 GOBP 分析。星号标注富集条目(q 值<0.05)。h. IPA 分析中从 TBI-aCD3 吞噬性小胶质细胞与 TBI-iso 吞噬性/非吞噬性小胶质细胞组(TBI 后 7 天及凋亡神经元注射后 4 小时)的 DEGs 筛选出的典型通路。i. TBI-aCD3(+P) vs TBI-iso(-P)中预测的上游调控因子。j. 实验设计与图 b 相似的吞噬功能检测。数据以箱线图呈现(最小值、最大值、四分位距、中位数),每组 n=5 只小鼠。 数据采用单因素方差分析(ANOVA)结合 Tukey 多重比较法进行统计分析。所有数据均为生物学重复样本,代表两项独立实验。
Extended Data Fig. 7. Nasal anti-CD3 increases expression of phagocytosis machinery at 16 hours post injection of apoptotic neurons.
扩展数据图 7. 鼻腔给予抗 CD3 单抗可提升注射凋亡神经元 16 小时后小胶质细胞吞噬机制相关蛋白的表达水平。
(a) Schematic presenting phagocytosis functional study. Created with BioRender.com. (b)
In-vivo phagocytosis functional experiment where mice were injected with labelled apoptotic neurons and sacrificed 16 h post injection. Gating strategy showing phagocytic positive microglia in TBI-Iso and TBI-aCD3 animals. Data shown as box plots (min, max, interquartile range, median) and n = 5 mice/group were used. Data was analyzed by two-sided unpaired Student’s t-test. (c) Clustered heatmap of select DEGs of aggregated samples for phagocytic ( + P) and non-phagocytic (-P) microglia at 7 days following TBI and 16 hours post-injection of apoptotic neurons using DESeq2 analysis (two-sided likelihood ratio test, n = 5-6 mice/group, FDR-corrected P < 0.05). Identified genes pertinent to microglial phagocytosis and related functions are visualized through bar plots with log2-fold changes in phagocytic TBI-aCD3 microglia compared to non-phagocytic TBI-Iso microglia. (d) GSEA analysis of GO Biological Process (BP) comparing phagocytic TBI-aCD3 microglia to phagocytic TBI-Iso microglia at 7 days following TBI and 16 hours post-injection of apoptotic neurons. NES, normalized enrichment score. (e) Bar plots of select microglial homeostatic and neurodegenerative microglia (MGnD) markers in phagocytic TBI-aCD3 microglia compared to phagocytic TBI-Iso microglia at 7 days following TBI and 16 hours post-injection of apoptotic neurons. DEGs indicated with an asterisk: FDR-corrected P < 0.05; DEGs indicated with “*P”: P < 0.05 (DESeq2 analysis, two-sided Wald test, n = 5 mice/group). (f) Gating strategy showing how phagocytic microglia cells were identified. All data are biological replicates and are representative from two independent experiments. n.s. = non-significant.
(a) 吞噬功能研究示意图。使用 BioRender.com 创建。(b) 体内吞噬功能实验:向小鼠注射标记的凋亡神经元,注射 16 小时后处死。门控策略显示 TBI-Iso 组和 TBI-aCD3 组中具有吞噬活性的小胶质细胞。数据以箱线图呈现(最小值、最大值、四分位距、中位数),每组 n=5 只小鼠。采用双尾非配对 Student t 检验分析数据。(c) 通过 DESeq2 分析(双尾似然比检验,每组 n=5-6 只小鼠,FDR 校正 P<0.05)获得的 TBI 后 7 天及凋亡神经元注射 16 小时后吞噬性(+P)与非吞噬性(-P)小胶质细胞差异表达基因(DEGs)聚类热图。与小胶质细胞吞噬功能相关的关键基因通过条形图展示,显示吞噬性 TBI-aCD3 小胶质细胞相较于非吞噬性 TBI-Iso 小胶质细胞的 log2 倍数变化。(d) TBI 后 7 天及凋亡神经元注射 16 小时时,吞噬性 TBI-aCD3 与吞噬性 TBI-Iso 小胶质细胞的 GO 生物过程(BP)基因集富集分析(GSEA)。NES:标准化富集分数。 (e) 条形图显示创伤性脑损伤(TBI)后 7 天及凋亡神经元注射 16 小时后,吞噬性 TBI-aCD3 小胶质细胞与吞噬性 TBI-Iso 小胶质细胞中选定的小胶质细胞稳态标志物及神经退行性小胶质细胞(MGnD)标志物的表达差异。标有星号的差异表达基因(DEGs):FDR 校正后 P 值<0.05;标有"*P"的 DEGs:P 值<0.05(DESeq2 分析,双侧 Wald 检验,每组 n=5 只小鼠)。(f) 展示吞噬性小胶质细胞鉴定策略的门控方案。所有数据均为生物学重复,结果来自两次独立实验。n.s.表示无统计学意义。
To investigate the role of IL-10 in the regulation of microglia phagocytic capacity after TBI, we repeated the in vivo microglia phagocytosis assay using IL-10 knockout (KO) mice (Fig. 4j). We found that the increased phagocytic capacity of TBI-aCD3 microglia at 4 h post-injection was significantly attenuated in the IL-10 KO TBI-aCD3 microglial group. Collectively, these findings demonstrate that nasal aCD3 treatment increases the phagocytic machinery of microglia after acute TBI, in an IL-10-dependent manner. In addition, we found that the phagocytic capacity of wild-type (WT) TBI-iso microglia was significantly reduced in the IL-10 KO TBI-iso microglia group, demonstrating the importance of IL-10 in microglial phagocytic function after TBI.
为探究 IL-10 在创伤性脑损伤(TBI)后调控小胶质细胞吞噬功能中的作用,我们使用 IL-10 基因敲除(KO)小鼠重复了体内小胶质细胞吞噬实验(图 4j )。研究发现,在 IL-10 KO TBI-aCD3 组中,注射后 4 小时 TBI-aCD3 小胶质细胞增强的吞噬能力显著减弱。这些结果共同表明,鼻腔给予 aCD3 通过 IL-10 依赖性机制增强了急性 TBI 后小胶质细胞的吞噬功能。此外,我们发现野生型(WT)TBI-iso 组小胶质细胞的吞噬能力在 IL-10 KO TBI-iso 组中显著降低,证实了 IL-10 对 TBI 后小胶质细胞吞噬功能的关键作用。
Nasal aCD3 ameliorates TBI outcomes via IL-10/IL-10R signaling in microglia
鼻腔给予 aCD3 通过小胶质细胞中 IL-10/IL-10R 信号通路改善 TBI 预后
IL-10 is a potent anti-inflammatory cytokine produced by Treg cells that acts on many cell types as a result of the presence of IL-10 receptor (IL-10R) on almost all hematopoietic cells81. IL-10 signaling is critical to maintain microglia under a homeostatic phenotype, because genetic depletion of IL-10 under proinflammatory conditions results in increased release of proinflammatory cytokines and chemokines82. We found a clear upregulation of the microglial IL-10-cytokine gene expression signature in TBI-aCD3 microglia group at 7 days post-CCI as compared to Sham-Iso and TBI-Iso controls (Fig. 5a). We also found that nasal aCD3 induced IL-10-secreting Treg cells (from day 3 to day 30 post-injury) (Fig. 5b and Extended Data Fig. 8a) and increased IL-10 expression in both microglia and brain tissue derived from the site of injury (Fig. 3h and Extended Data Fig. 6c). We then investigated whether IL-10 played a role in the beneficial effects of nasal aCD3 by administering anti-IL-10R (aIL-10R)-blocking antibody intraperitoneally (i.p.) every 3 d post-injury (Fig. 5c) and investigating the behavioral outcomes and BBB disruption in sham-iso, TBI-iso, TBI-aCD3 and TBI-aCD3+aIL-10R groups. We found that the improvements in BBB disruption (Fig. 5d) and motor coordination functions, spatial memory and anxiety-like behavior observed in TBI-aCD3 were abrogated by blocking IL-10R (TBI-aCD3+aIL-10R group) (Fig. 5e).
IL-10 是一种由 T reg 细胞产生的强效抗炎细胞因子,由于几乎所有造血细胞 81 表面都存在 IL-10 受体(IL-10R),其可作用于多种细胞类型。IL-10 信号传导对维持小胶质细胞稳态表型至关重要,因为在促炎条件下 IL-10 基因缺失会导致促炎细胞因子和趋化因子释放增加 82 。我们发现,与 Sham-Iso 和 TBI-Iso 对照组相比,CCI 术后 7 天的 TBI-aCD3 小胶质细胞组中,小胶质细胞 IL-10 细胞因子基因表达特征明显上调(图 5a )。研究还发现鼻腔给予 aCD3 可诱导 IL-10 分泌型 T reg 细胞(从损伤后第 3 天持续至第 30 天)(图 5b 及扩展数据图 8a ),并提高损伤部位小胶质细胞和脑组织中的 IL-10 表达(图 3h 及扩展数据图 6c )。随后我们通过腹腔注射(i.p.)抗 IL-10R(aIL-10R)阻断抗体(每 3 天一次)来研究 IL-10 是否参与鼻腔 aCD3 的治疗作用(图 5c )并研究假手术组(sham-iso)、创伤性脑损伤组(TBI-iso)、抗 CD3 单抗治疗组(TBI-aCD3)及抗 CD3 单抗联合 IL-10 受体阻断组(TBI-aCD3+aIL-10R)的行为学结果与血脑屏障破坏情况。我们发现,抗 CD3 单抗治疗组(TBI-aCD3)在改善血脑屏障破坏(图 5d )、运动协调功能、空间记忆及焦虑样行为方面的效果,均被 IL-10 受体阻断剂(TBI-aCD3+aIL-10R 组)所抵消(图 5e )。
Fig. 5. Nasal aCD3 ameliorates TBI microglial inflammation and functional outcomes in an IL-10-dependent manner.
图 5. 鼻腔给予 aCD3 通过 IL-10 依赖性途径改善 TBI 小胶质细胞炎症并促进功能恢复
a, Heatmap of genes of aggregated samples involved in the IL-10 pathway for microglia at 7 and 30 d post-TBI. Genes identified with an FDR-corrected P < 0.05 using DESeq2 analysis are emboldened and indicated by an asterisk (two-sided LRT, n = 4 mice per group). Genes were identified from the literature109. b, IL-10 expression (flow cytometry) in different cells at different time points post-TBI and treatment in the ipsilateral hemisphere (sham-iso n = 4, TBI-iso n = 6, TBI-aCD3 n = 6), analyzed by one-way ANOVA with Tukey’s multiple comparisons for individual time points. Data are shown as mean ± s.e.m. NK, natural killer cell. c, Experimental timeline of anti-IL-10R-blocking mAbs (aIL-10R) (created with BioRender.com). d, Dextran 70-kDa (green) for measurement of BBB permeability (3 d post-TBI). Scale bars, 1,000 μm. Data are shown as mean ± s.e.m. (sham-iso n = 3, n = 6 for the rest of the groups), analyzed by one-way ANOVA with Tukey’s multiple comparisons. e, Behavioral testing (rotarod, MWM, probe trial, OF for anxiety-like behavior). The MWM was analyzed by two-factor, repeated-measures, two-way ANOVA (group × time) and the others by one-way ANOVA with Tukey’s multiple comparisons. Data are shown as mean ± s.e.m. (n = 8 mice per group). f, Clustered heatmap DEGs at 30 d post-TBI identified using DESeq2 analysis (two-sided LRT, n = 4–8 mice per group, FDR-corrected P < 0.05). The microglial data at 30 d post-TBI from Fig. 3c were integrated. g, Experimental timeline for the microglia-specific IL-10ra KO (created with BioRender.com). h, Behavioral testing of rotarod and Y-maze assessed between the groups. Data are shown as mean ± s.e.m. (WT sham-nasal-iso n = 6, Tmem119WT:IL-10raFlx/Flx-TBI-Nasal-aCD3 n = 8, Tmem119CreETR2:IL-10raFlx/Flx-TBI-Nasal-aCD3 n = 6, Tmem119WT:IL-10raFlx/Flx-TBI-nasal-iso n = 8, Tmem119 CreETR2:IL-10raFlx/Flx-TBI-nasal-iso n = 6). Analysis was by one-way ANOVA with Tukey’s multiple comparisons. i, Phagocytosis experiment with a similar design to Fig. 4b. Data are shown as box plots (min., max., IQR, median; n = 5 mice per group), analyzed by one-way ANOVA with Tukey’s multiple comparisons. All data are biological replicates and represent two independent experiments.
a. TBI 后 7 天和 30 天小胶质细胞 IL-10 通路相关基因的样本聚合热图。经 DESeq2 分析(双侧 LRT,每组 n=4 只小鼠)确定 FDR 校正 P 值<0.05 的基因以加粗字体标出并附星号标记。基因筛选依据文献 109 。b. 采用流式细胞术检测 TBI 后不同时间点及治疗组损伤同侧半球 IL-10 表达(假手术组同型对照 n=4,TBI 组同型对照 n=6,TBI-aCD3 组 n=6),各时间点数据通过单因素方差分析结合 Tukey 多重比较检验。数据以均值±标准误表示。NK:自然杀伤细胞。c. 抗 IL-10R 阻断单抗(aIL-10R)实验时间轴(使用 BioRender.com 制作)。d. 70-kDa 右旋糖酐(绿色)检测 TBI 后 3 天血脑屏障通透性。比例尺:1,000 μm。数据以均值±标准误表示(假手术组同型对照 n=3,其余组 n=6),采用单因素方差分析结合 Tukey 多重比较检验。e. 行为学测试(转棒实验、Morris 水迷宫、探测试验、旷场焦虑样行为检测)。Morris 水迷宫采用双因素重复测量双因素方差分析(组别×时间),其余测试采用单因素方差分析结合 Tukey 多重比较检验。 数据以均值±标准误表示(每组 n=8 只小鼠)。f、采用 DESeq2 分析鉴定的 TBI 后 30 天差异表达基因聚类热图(双侧似然比检验,每组 n=4-8 只小鼠,FDR 校正 P<0.05)。整合了图 3c 中 TBI 后 30 天的小胶质细胞数据。g、小胶质细胞特异性 IL-10ra 敲除实验时间线(使用 BioRender.com 创建)。h、各组间旋转棒和 Y 迷宫行为学测试结果。数据以均值±标准误表示(WT 假手术-鼻腔同型对照 n=6,Tmem119 WT :IL-10ra Flx/Flx -TBI-鼻腔 aCD3 n=8,Tmem119 CreETR2 :IL-10ra Flx/Flx- TBI-鼻腔 aCD3 n=6,Tmem119 WT :IL-10ra Flx/Flx -TBI-鼻腔同型对照 n=8,Tmem119 CreETR2 :IL-10ra Flx/Flx- TBI-鼻腔同型对照 n=6)。采用单因素方差分析及 Tukey 多重比较。i、吞噬实验设计与图 4b 类似。数据以箱线图表示(最小值、最大值、四分位距、中位数;每组 n=5 只小鼠),采用单因素方差分析及 Tukey 多重比较。所有数据均为生物学重复,代表两次独立实验。
Extended Data Fig. 8. Gating strategy of IL-10 production, tamoxifen induced microglia specific IL10ra reduction in gene expression, and FoxP3(GFP)+ gating strategy.
扩展数据图 8. IL-10 产生的门控策略、他莫昔芬诱导的小胶质细胞特异性 IL10ra 基因表达降低,以及 FoxP3(GFP)+门控策略。
(a) Gating strategy for IL-10 expression on CD4 + , FoxP3 + , 4D4+ microglia, NK1.1 + , Ly6C + , and Ly6G+ cell and their fluorescence minus one (FMO) control. (b) Bar plot of Quantitative PCR of microglia sorted from the ipsilateral hemisphere at 7 days post TBI for microglia specific IL-10ra knockout Tmem119CreETR2:IL-10raFlx/Flx and their littermate controls Tmem119WT:IL-10raFlx/Flx. Mice were treated with tamoxifen for 5 straight days and were given a 2-week rest period before TBI. Expression was normalized to GAPDH. Data shown as mean ± SEM, n = 3 mice/group. Data was analyzed by two-sided unpaired Student’s t-test. The data are biological replicates and are representative from three independent experiments. (c) Gating strategy showing CD4+FoxP3 GFP+ and the population of CD4+FoxP3 GFP- from the spleen/cLN that was selected for the adoptive transfer experiments in Fig. 6 and Extended Data Fig. 9.
(a) CD4+、FoxP3+、4D4+小胶质细胞、NK1.1+、Ly6C+及 Ly6G+细胞中 IL-10 表达的设门策略及其荧光减一(FMO)对照。(b) TBI 后 7 天从同侧半球分选的小胶质细胞定量 PCR 柱状图,显示小胶质细胞特异性 IL-10ra 敲除组 Tmem119 CreETR2 :IL-10ra Flx/Flx 及其同窝对照 Tmem119 WT :IL-10ra Flx/Flx 。小鼠连续 5 天接受他莫昔芬处理,TBI 前休息 2 周。表达量以 GAPDH 标准化。数据显示为均值±SEM,n=3 只/组。采用双侧非配对 Student t 检验分析。数据为生物学重复,代表三次独立实验。(c) 显示 CD4 + FoxP3 GFP + 的设门策略及来自脾脏/颈淋巴结的 CD4 + FoxP3 GFP - 细胞群,该群体被选用于图 6 和扩展数据图 9 中的过继转移实验。
We next investigated the impact of blocking IL-10R on the microglial inflammatory transcriptomic profile by performing RNA-seq on sorted microglia from the ipsilateral hemisphere of the brain (Extended Data Fig. 6a) at 1 month post-CCI (Fig. 3b and Supplementary Table 4). We found that the modulatory effect of nasal aCD3 on microglia was abrogated by blocking IL-10 as shown in the microglial heatmap signature (Fig. 5f). At 1 month post-CCI, similar to the transcriptomic signature of TBI-iso control, microglia from the TBI-aCD3+aIL-10R group had a more proinflammatory profile compared with sham-iso and the TBI-aCD3 group and was associated with decreased expression of homeostatic markers such as Mertk, Tgfbr2, Atp8a2, and Adgrg1 (Fig. 5f and Supplementary Table 4).
我们随后通过 RNA 测序研究了阻断 IL-10R 对损伤同侧大脑半球小胶质细胞炎症转录组特征的影响(扩展数据图 6a ),检测时间为 CCI 术后 1 个月(图 3b 和补充表 4 )。小胶质细胞热图特征显示(图 5f ),鼻腔给予 aCD3 对小胶质细胞的调节作用会因 IL-10 阻断而消除。CCI 术后 1 个月,与 TBI-iso 对照组相似,TBI-aCD3+aIL-10R 组的小胶质细胞较假手术组和 TBI-aCD3 组表现出更强的促炎特征,同时伴随稳态标志物(如 Mertk、Tgfbr2、Atp8a2 和 Adgrg1)表达水平下降(图 5f 和补充表 4 )。
We hypothesized that the beneficial effect of the Treg cells induced by nasal aCD3 was dependent on IL10R signaling in microglia. We thus investigated this hypothesis by using IL-10Rflox/floxTmem119CreETR2 conditional and tamoxifen-induced KO mice and littermate controls (Extended Data Fig. 8b). We investigated the effects of microglial IL-10R ablation on the behavioral and microglial phagocytic capacity at 7 d post-TBI (Fig. 5g). We found that tamoxifen-treated TBI-aCD3-IL-10Rflox/floxTmem119CreETR2 mice exhibited worse motor and cognitive outcomes (Fig. 5h) and reduced microglial phagocytic capacity (Fig. 5i) compared with tamoxifen-treated TBI-aCD3 littermate controls, further supporting the role for IL-10 in modulating the microglial gene signature after nasal aCD3 treatment. Taken together, these data clearly demonstrate a critical role for IL-10/IL-10R signaling in augmenting microglial phagocytic capacity and ameliorating disease in TBI.
我们假设鼻腔给予 aCD3 诱导的 T reg 细胞的神经保护作用依赖于小胶质细胞中的 IL10R 信号通路。为此,我们使用 IL-10R flox/flox Tmem119 CreETR2 条件性敲除和他莫昔芬诱导的基因敲除小鼠及其同窝对照进行验证(扩展数据图 8b )。研究发现在 TBI 后第 7 天,小胶质细胞 IL-10R 缺失对行为学表现和小胶质细胞吞噬功能的影响(图 5g )。与他莫昔芬处理的 TBI-aCD3 同窝对照组相比,他莫昔芬处理的 TBI-aCD3-IL-10R flox/flox Tmem119 CreETR2 小鼠表现出更差的运动及认知功能(图 5h )和降低的小胶质细胞吞噬能力(图 5i ),这进一步证实了 IL-10 在鼻腔 aCD3 治疗后调节小胶质细胞基因特征中的作用。综上所述,这些数据明确表明 IL-10/IL-10R 信号通路在增强小胶质细胞吞噬能力和改善 TBI 病理过程中具有关键作用。
Nasal aCD3-induced CD4+FoxP3+ Treg cells ameliorate neuroinflammation and TBI outcomes
鼻腔给予 aCD3 诱导的 CD4 + FoxP3 + T reg 细胞可改善神经炎症及创伤性脑损伤预后
In the days to weeks after TBI, lymphocytes appear at the lesion site and both effector T cells and Treg cells infiltrate the injured brain83–86. We found that nasal aCD3 expanded FoxP3+ Treg cells at the injury site from day 3 to day 30 post-injury compared with the TBI-iso controls (Fig. 2b) and that nasal aCD3 improved behavioral and neuropathological outcomes of TBI by decreasing microglial inflammation (Figs. 1b and 3c). Thus, we next investigated whether these beneficial outcomes were dependent on Treg cells by employing multiple experimental approaches including adoptive transfer experiments (discussed here) and an ex vivo Treg cell–microglia transwell co-culture system and Treg cell depletion experiments (discussed below). In the experiments involving adoptive transfer, we found that the adoptive transfer of CD4+FoxP3+ Treg cells from aCD3-treated mice improved motor function and coordination and restored spatial memory when compared with mice that received either CD4+FoxP3+ Treg cells from TBI-iso mice or CD4+ T cells depleted of Treg cells from anti-CD3-treated mice at 7 d post-TBI (Fig. 6b). We then performed RNA-seq on microglia isolated from the ipsilateral hemisphere of TBI-aCD3-FoxP3+, TBI-iso-Foxp3+ and TBI-aCD3-FoxP3− green fluorescent protein (GFP) groups at 7 d post-TBI (Supplementary Table 5). We found that TBI-aCD3-FoxP3+ microglia had a distinct transcriptomic profile when compared with other groups (Fig. 6c). GSEA analysis comparing TBI-aCD3-FoxP3+ microglia with TBI-aCD3-FoxP3− microglia demonstrated that the TBI-aCD3-FoxP3+ microglia were associated with downregulation of several proinflammatory pathways including interferon (IFN)γ and IFNα (Fig. 6d). IPA revealed IL-10Ra as one of the top activated upstream regulators and IL-1b as one of the top inhibited upstream regulators in TBI-aCD3-FoxP3+ microglia compared with TBI-aCD3-FoxP3− microglia (Fig. 6e). IFNγ was one of the most downregulated regulators in TBI-aCD3-FoxP3+ microglia compared with TBI-iso-FoxP3+ microglia (Fig. 6f,g). Consistent with the microglial transcriptomic data, RT–qPCR of TBI-aCD3-FoxP3+, microglia from the lesion site showed reduction in proinflammatory cytokines (Inf-γ, Cd14 and Tnf) and increased expression of homeostatic (Cd206) and anti-inflammatory cytokines (such as Il10) compared with the two other groups studied (Fig. 6h). Importantly, we also found that adoptive transfer of CD4+FoxP3+ Treg cells from aCD3-treated mice increased microglial phagocytic capacity at 7 d post-injury compared with the two other groups (Fig. 6i). The microglial phagocytic capacity was also increased in mice that received CD4+FoxP3+ Treg cells from TBI-iso-mice compared with mice that received FoxP3− cells from aCD3-treated mice, indicating the importance of CD4+FoxP3+ Treg cells in increasing microglial phagocytic function after TBI.
在创伤性脑损伤(TBI)后的数天至数周内,淋巴细胞出现在病变部位,效应 T 细胞和调节性 T 细胞(Treg)均会浸润受损脑组织。我们发现,与 TBI-iso 对照组相比,鼻腔给予 aCD3 单抗能在损伤后第 3 天至第 30 天期间促进损伤部位 FoxP3+ Treg 细胞的扩增(图 4),并通过减轻小胶质细胞炎症反应改善 TBI 的行为学和神经病理学结局(图 5 和图 6)。因此,我们随后采用多种实验方法(包括下文讨论的过继转移实验、离体 Treg-小胶质细胞 Transwell 共培养系统及 Treg 细胞清除实验)来验证这些有益效应是否依赖于 Treg 细胞。在过继转移实验中,我们发现:与接受 TBI-iso 小鼠 CD4+FoxP3+ Treg 细胞或 aCD3 处理小鼠中清除 Treg 的 CD4+ T 细胞的受体鼠相比,移植 aCD3 处理小鼠 CD4+FoxP3+ Treg 细胞能在 TBI 后 7 天显著改善运动功能、协调能力并恢复空间记忆(图 18)。 随后我们对创伤性脑损伤后 7 天时从 TBI-aCD3-FoxP3 + 、TBI-iso-Foxp3 + 和 TBI-aCD3-FoxP3 − 绿色荧光蛋白(GFP)组同侧半球分离的小胶质细胞进行 RNA 测序(补充表 5 )。研究发现 TBI-aCD3-FoxP3 + 小胶质细胞与其他组相比具有独特的转录组特征(图 6c )。通过 GSEA 分析比较 TBI-aCD3-FoxP3 + 与 TBI-aCD3-FoxP3 − 小胶质细胞显示,TBI-aCD3-FoxP3 + 小胶质细胞中包括干扰素(IFN)γ和 IFNα在内的多个促炎通路表达下调(图 6d )。IPA 分析表明,与 TBI-aCD3-FoxP3 − 相比,IL-10Ra 是 TBI-aCD3-FoxP3 + 小胶质细胞中最显著激活的上游调节因子之一,而 IL-1b 则是受抑制程度最高的上游调节因子之一(图 6e )。与 TBI-iso-FoxP3 + 组相比,IFNγ是 TBI-aCD3-FoxP3 + 小胶质细胞中下调最显著的调节因子(图 6f,g )。 与小胶质细胞转录组数据一致,RT-qPCR 分析显示,与其他两组相比,TBI-aCD3-FoxP3 处理组病灶区小胶质细胞的促炎细胞因子(Inf-γ、Cd14 和 Tnf)表达降低,而稳态标志物(Cd206)和抗炎细胞因子(如 Il10)表达升高(图 6h )。值得注意的是,我们还发现,与其他两组相比,在损伤后 7 天时,接受 aCD3 处理小鼠来源的 CD4 + FoxP3 + T reg 细胞过继转移可增强小胶质细胞的吞噬能力(图 6i )。与接受 aCD3 处理小鼠 FoxP3 − 细胞组相比,接受 TBI-同型对照小鼠 CD4 + FoxP3 + T reg 细胞组的小胶质细胞吞噬能力也有所提升,这表明 CD4 + FoxP3 + T reg 细胞对 TBI 后增强小胶质细胞吞噬功能具有重要作用。
Fig. 6. Nasal aCD3-induced CD4+FoxP3+ Treg cells attenuate the CNS inflammatory response, increase microglial phagocytosis and improve behavioral outcomes after TBI.
图 6. 鼻腔给予 aCD3 诱导的 CD4 + FoxP3 + T reg 细胞可减轻 TBI 后中枢神经系统炎症反应、增强小胶质细胞吞噬功能并改善行为学结局
a, Experimental timeline of adoptive transfer (created with BioRender.com). b, Behavioral testing of rotarod and Y-maze assessed. Data are shown as mean ± s.e.m. (sham-DPBS n = 6, TBI-aCD3-FoxP3 n = 8, TBI-iso FoxP3 n = 8, TBI-aCD3-FoxP3−
n = 8) and analyzed by one-way ANOVA with Tukey’s multiple comparisons. c, Clustered heatmap of unique and shared DEGs of aggregated samples from microglia at 7 d post-TBI on the following comparisons: TBI-iso FoxP3 versus TBI-aCD3-FoxP3− and TBI-aCD3-FoxP3 versus TBI-aCD3-FoxP3−. Clusters were functionally annotated using enriched GOBP terms (q-value < 0.05). DEGs were identified using DESeq2 analysis (two-sided Wald’s test, n = 3 mice per group, FDR-corrected P < 0.05). d, GSEA analysis of Hallmark pathways at 7 d post-TBI for the following: TBI-iso FoxP3 versus TBI-aCD3-FoxP3− and TBI-aCD3-FoxP3 versus TBI-aCD3-FoxP3−. The asreisk indicates enriched terms (q-value < 0.05). e, Predicted upstream regulators (IPA analysis) in TBI-aCD3-FoxP3 versus TBI-aCD3-FoxP3−. f, GSEA analysis of Hallmark pathways comparing TBI-aCD3-FoxP3 versus TBI-iso FoxP3. g, Predicted upstream regulator (IPA analysis) in TBI-aCD3-FoxP3 versus TBI-Iso FoxP3. h, RT–qPCR of microglia sorted from the ipsilateral hemisphere 7 d post-TBI. Expression was normalized to GAPDH and presented relative to sham-DPBS mice. Data are shown as mean ± s.e.m., n = 3–4 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. i, Phagocytosis experiment with similar design to Fig. 4b. Data are shown as box plots (min., max., IQR, median), n = 4 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. j, Schematic representing microglia and Treg cell transwell co-culture (created with BioRender.com). Microglia were isolated 24 h post-TBI and cultured with FoxP3 Treg cells from either nasal aCD3 or isotype-treated TBI mice for 7 d. j, RT–qPCR of microglia, expression normalized to GAPDH. Data are shown as mean ± s.e.m., n = 4 conditions per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. All data and conditions are biological replicates and represent two independent experiments.
a、过继转移实验时间轴(使用 BioRender.com 创建)。b、转棒实验和 Y 迷宫行为学测试评估结果。数据以均值±标准误表示(假手术-DPBS 组 n=6,TBI-aCD3-FoxP3 组 n=8,TBI-iso FoxP3 组 n=8,TBI-aCD3-FoxP3 − 组 n=8),采用单因素方差分析及 Tukey 多重比较进行统计分析。c、创伤性脑损伤后 7 天小胶质细胞样本差异表达基因(DEGs)的聚类热图,比较组包括:TBI-iso FoxP3 组 vs TBI-aCD3-FoxP3 − 组,以及 TBI-aCD3-FoxP3 组 vs TBI-aCD3-FoxP3 − 组。聚类结果采用富集的 GOBP 术语进行功能注释(q 值<0.05)。DEGs 通过 DESeq2 分析鉴定(双侧 Wald 检验,每组 n=3 只小鼠,FDR 校正 P 值<0.05)。d、创伤性脑损伤后 7 天 Hallmark 通路的 GSEA 分析,比较组包括:TBI-iso FoxP3 组 vs TBI-aCD3-FoxP3 − 组,以及 TBI-aCD3-FoxP3 组 vs TBI-aCD3-FoxP3 − 组。星号标注富集术语(q 值<0.05)。e、TBI-aCD3-FoxP3 组 vs TBI-aCD3-FoxP3 − 组的预测上游调控因子(IPA 分析结果)。f、TBI-aCD3-FoxP3 组 vs TBI-iso FoxP3 组的 Hallmark 通路 GSEA 分析。g、TBI-aCD3-FoxP3 组 vs TBI-Iso FoxP3 组的预测上游调控因子(IPA 分析结果)。 h,创伤性脑损伤(TBI)后 7 天对同侧半球分选小胶质细胞进行实时定量 PCR 分析。表达量以 GAPDH 为内参,数据以假手术-DPBS 组为基准呈现。结果以均值±标准误表示,每组 n=3-4 只小鼠,采用单因素方差分析及 Tukey 多重比较检验。i,吞噬实验设计与图 4b 类似。数据以箱线图(最小值、最大值、四分位距、中位数)展示,每组 n=4 只小鼠,采用单因素方差分析及 Tukey 多重比较检验。j,示意图展示小胶质细胞与 T reg 细胞 Transwell 共培养体系(使用 BioRender.com 绘制)。小胶质细胞于 TBI 后 24 小时分离,并与鼻内 aCD3 或同型对照处理的 TBI 小鼠 FoxP3 T reg 细胞共培养 7 天。j,小胶质细胞实时定量 PCR 结果,表达量以 GAPDH 标准化。数据以均值±标准误表示,每组 n=4 个条件,采用单因素方差分析及 Tukey 多重比较检验。所有数据及条件均为生物学重复,代表两次独立实验。
To assess the beneficial effects of Treg cells on behavioral outcomes and microglial inflammation at more chronic time points (30 d) post-TBI, we employed an alternative approach where total splenic T cells (CD45.2+CD4+) isolated from TBI-iso (iso-total CD4+) and TBI-nasal aCD3-treated mice (aCD3-total CD4+) and CD45.2+CD4+FoxP3− GFP cells isolated from aCD3-treated animals (aCD3-FoxP3− GFP) post-CCI were transferred i.p. into untreated but CCI-injured congenic CD45.1-expressing mice (Extended Data Figs. 8c and 9a). Adoptive transfer was performed at three time points post-CCI with each mouse receiving 2.5 × 106 cells per injection. We found improvement in motor function and coordination, restoration of spatial memory and increased time spent in the target quadrant during the probe trial in mice that received total CD4+ T cells from aCD3-treated mice compared with mice that received CD4+ T cells depleted of Treg cells at 30 d post-TBI (Extended Data Fig. 9b). We performed flow cytometric analyses to track adoptively transferred cells in recipient mice by transferring cells from CD45.2 mice into CD45.1 mice and found CD45.2 transferred cells in all organs analyzed (Extended Data Fig. 9c). We then performed RNA-seq on microglia isolated from the ipsilateral hemisphere of iso-total CD4+, aCD3-total CD4+ and aCD3-FoxP3− GFP groups at 30 d post-TBI (Supplementary Table 5). A heatmap signature showed a distinct microglial transcriptomic signature of aCD3-total CD4+ compared with iso-total CD4+ and aCD3-FoxP3− GFP groups at 30 d post-CCI (Extended Data Fig. 9d). GSEA pathway analysis demonstrated that the aCD3-total CD4+ group was associated with the upregulation of neuron development pathways and the downregulation of several proinflammatory pathways involved in innate and adaptive immune responses, immune effector processes and antigen presentation compared with the iso-total CD4+ group, all of which were not notably upregulated in the aCD3-FoxP3− GFP group (Extended Data Fig. 9e). Similarly, the aCD3-total CD4+ group was associated with a downregulation of the innate immune response pathway when compared with the aCD3-FoxP3− GFP group (Extended Data Fig. 9f,g), where IPA analysis demonstrated IL-10 as a top activated upstream regulator and IFNγ as a top inhibited upstream regulator (Extended Data Fig. 9h). Consistent with the microglial transcriptomic data, RT–qPCR of the ipsilateral hemisphere showed an increase in the expression of several anti-inflammatory cytokines (Il10 and Il2), and growth factors including Gdnf at 1 month post-CCI in the aCD3-total CD4+ group compared with the iso-total CD4+ and aCD3-FoxP3− GFP groups (Extended Data Fig. 9i). Taken together, these data demonstrate a critical role for FoxP3+ Treg cells in augmenting microglia phagocytic capacity and ameliorating disease in TBI.
为评估 T reg 细胞在创伤性脑损伤(TBI)后较长期时间点(30 天)对行为学结果和小胶质细胞炎症的改善作用,我们采用替代方案:将分别从 TBI 同型对照组(iso-total CD4 + )和鼻腔给予 aCD3 治疗的 TBI 小鼠(aCD3-total CD4 + )分离的脾脏总 T 细胞(CD45.2 + CD4 + ),以及从 aCD3 治疗动物分离的 CD45.2 + CD4 + FoxP3 − GFP 细胞(aCD3-FoxP3 − GFP),通过腹腔移植至未经处理但接受 CCI 损伤的 CD45.1 同源基因小鼠体内(扩展数据图 8c 和 9a )。移植在 CCI 术后三个时间点进行,每次注射 2.5×10 6 个细胞。研究发现,与接受去除 T reg 细胞的 CD4 + T 细胞移植组相比,接受 aCD3 治疗小鼠来源的总 CD4 + T 细胞移植组在 TBI 后 30 天表现出运动功能与协调性改善、空间记忆恢复,且在探测试验中目标象限停留时间显著延长(扩展数据图 9b )。 我们通过流式细胞术分析追踪过继转移细胞,将 CD45.2 小鼠的细胞转移至 CD45.1 受体小鼠后,在所有检测器官中均发现 CD45.2 来源的转移细胞(扩展数据图 9c )。随后我们对创伤性脑损伤(TBI)后 30 天从同侧半球分离的小胶质细胞进行 RNA 测序,样本来自 iso-total CD4 + 组、aCD3-total CD4 + 组和 aCD3-FoxP3 − GFP 组(补充表 5 )。热图特征显示,在 CCI 术后 30 天,aCD3-total CD4 + 组与 iso-total CD4 + 组及 aCD3-FoxP3 − GFP 组相比具有显著不同的小胶质细胞转录组特征(扩展数据图 9d )。GSEA 通路分析表明,与 iso-total CD4 + 组相比,aCD3-total CD4 + 组与神经元发育通路的上调相关,同时下调了涉及先天性和适应性免疫应答、免疫效应过程及抗原呈递的多个促炎通路,而这些通路在 aCD3-FoxP3 − GFP 组均未显著上调(扩展数据图 9e )。 同样,与 aCD3-FoxP3 − GFP 组相比,aCD3-total CD4 + 组显示出先天免疫反应通路的下调(扩展数据图 9f,g ),其中 IPA 分析表明 IL-10 是最活跃的上游调节因子,而 IFNγ则是受抑制程度最高的上游调节因子(扩展数据图 9h )。与小胶质细胞转录组数据一致,RT-qPCR 显示在 CCI 后 1 个月,aCD3-total CD4 + 组损伤同侧大脑半球中多种抗炎细胞因子(Il10 和 Il2)及神经营养因子(如 Gdnf)的表达较 iso-total CD4 + 和 aCD3-FoxP3 − GFP 组显著增加(扩展数据图 9i )。这些数据共同表明 FoxP3 + T reg 细胞在增强小胶质细胞吞噬能力及改善 TBI 病理过程中发挥关键作用。
Extended Data Fig. 9. Nasal anti-CD3 induced Total CD4 + T-cells ameliorate microglial response and improved behavioral outcomes at chronic TBI.
扩展数据图 9. 鼻腔给予抗 CD3 诱导的总 CD4+T 细胞可改善慢性 TBI 中小胶质细胞反应并促进行为学恢复。
(a) Schematic representing experimental timeline of adoptive transfer experiment. Created with BioRender.com. (b) Behavioral testing of rotarod, Morris water maze, probe trial, and anxiety like behavior (measured by the open field) was assessed between WT Sham (DPBS treated, baseline), Iso-total CD4+, aCD3-total CD4+, and aCD3-FoxP3-GFP groups. Morris water maze analyzed by two-factor repeated measures two-way ANOVA (group x time); others by one-way ANOVA with Tukey’s multiple comparisons. Data shown as mean ± SEM. (c) Visual representing an experiment where splenic CD4+ cells from 7 days treated TBI (FoxP3-GFP) animals were injected into untreated, but TBI-injured (CD45.1) animals and the %CD45.2 cells were analyzed by fluorescence-activated cell sorting (FACS) at 3 days after injection. Flow cytometry gating of brain, cervical lymph node, and spleen of (CD45.1) animals showing the percent of CD45.2 cell infiltration. n = 5 mice and the brain was a pool of 5 ipsilateral hemispheres. Created with BioRender.com. (d) Heatmap of DEGs from microglia 30 days following TBI and adoptive transfer identified using DESeq2 analysis (two-sided likelihood ratio test, n = 4 mice/group, FDR-corrected P < 0.05). Clusters of genes were functionally annotated using enriched GO Biological Process (BP) terms (q-value < 0.05). (e) GSEA analysis of GO Biological Process (BP) 30 days post-TBI based on the following comparisons: aCD3-Total CD4+ vs. Iso-Total CD4+, and aCD3-FoxP3-GFP vs. Iso-total CD4+. Asterisk (*) indicates enriched terms (q-value < 0.05). NES, normalized enrichment score; NS, not significant. (f) Volcano plot of DEGs in aCD3-total CD4+ vs. aCD3-FoxP3-GFP 30 days post-TBI identified using DESeq2 (two-sided Wald test, n = 4 mice/group). Labeled genes have an FDR-corrected P < 0.05. (g) GSEA analysis of GO Biological Process (BP) comparing aCD3-total CD4+ vs. aCD3-FoxP3-GFP 30 days post-TBI. NES, normalized enrichment score. (h) Predicted top upstream regulators using IPA analysis based on DEGs in aCD3-total CD4+ vs. aCD3-FoxP3-GFP 30 days post-TBI. (i) RT- qPCR of ipsilateral hemisphere 30 days post-TBI and expression normalized to GAPDH. Data shown as mean ± SEM, n = 5 mice/group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. All data are biological replicates and are representative from two independent experiments. n.s. = non-significant.
(a) 实验时间轴示意图展示过继性转移实验流程。使用 BioRender.com 软件绘制。(b) 行为学测试包括转棒实验、莫里斯水迷宫、探针测试及焦虑样行为(通过旷场实验评估),比较了 WT 假手术组(DPBS 处理基线)、Iso-total CD4 + 组、aCD3-total CD4 + 组与 aCD3-FoxP3 - GFP 组。莫里斯水迷宫采用双因素重复测量双向方差分析(组别×时间),其余测试采用单因素方差分析结合 Tukey 多重比较。数据以均值±标准误表示。(c) 实验示意图显示:将经 7 天处理的 TBI(FoxP3-GFP)动物脾脏 CD4 + 细胞注射入未处理但遭受 TBI 损伤的(CD45.1)动物体内,注射 3 天后通过流式细胞术(FACS)分析 CD45.2 细胞百分比。流式细胞术门控分析显示(CD45.1)动物脑组织、颈淋巴结及脾脏中 CD45.2 细胞浸润比例。n=5 只小鼠,脑组织样本为 5 个同侧半球混合。使用 BioRender.com 软件绘制。 (d) TBI 及过继转移后 30 天小胶质细胞差异表达基因(DEGs)热图,采用 DESeq2 分析鉴定(双侧似然比检验,n=4 只小鼠/组,FDR 校正 P<0.05)。基因簇通过富集的 GO 生物过程(BP)术语进行功能注释(q 值<0.05)。(e) TBI 后 30 天 GO 生物过程(BP)的 GSEA 分析,基于以下比较组:aCD3-Total CD4 + vs. Iso-Total CD4 + ,以及 aCD3-FoxP3 - GFP vs. Iso-total CD4 + 。星号(*)标注富集术语(q 值<0.05)。NES,标准化富集分数;NS,无统计学意义。(f) TBI 后 30 天 aCD3-total CD4 + vs. aCD3-FoxP3 - GFP 的 DEGs 火山图,采用 DESeq2 鉴定(双侧 Wald 检验,n=4 只小鼠/组)。标注基因的 FDR 校正 P<0.05。(g) TBI 后 30 天 aCD3-total CD4 + vs. aCD3-FoxP3 - GFP 的 GO 生物过程(BP)GSEA 分析。NES,标准化富集分数。(h) 基于 TBI 后 30 天 aCD3-total CD4 + vs. aCD3-FoxP3 - GFP 的 DEGs,通过 IPA 分析预测的顶级上游调控因子。(i) TBI 后 30 天损伤同侧大脑半球的 RT-qPCR 检测结果,表达量经 GAPDH 标准化。 数据以均值±标准误表示,每组 n=5 只小鼠,采用单因素方差分析及 Tukey 多重比较法进行统计分析。所有数据均为生物学重复样本,结果来自两次独立实验。n.s.表示无统计学差异。
CD4+Foxp3+ Treg cells suppress microglial inflammatory and homeostatic markers in vitro
CD4 + Foxp3 + T reg 细胞在体外抑制小胶质细胞的炎症和稳态标志物
To further investigate the interaction between Treg cells and microglia after TBI, we employed an ex vivo transwell co-culture system in which microglia were isolated from the ipsilateral hemisphere of CCI mice 24 h after injury and Treg cells were isolated from spleens of a separate cohort of mice subjected to CCI and treated with nasal aCD3 or isotype control for 7 d (Fig. 6j). Microglia were placed in the lower chamber and Treg cells in the upper chamber and we performed RT–qPCR of microglia 72 h after incubation. We found an increase in anti-inflammatory and homeostatic markers (Il10, Cx3cr1 and Cd206) and a reduction in the proinflammatory marker Il1b in the TBI-aCD3 microglia, which are consistent with our in vivo microglial transcriptomic data at 7 d post-TBI. This effect was lost by blocking IL-10 in vitro which is also consistent with what we observed following in vivo Il-10 neutralization (Fig. 5).
为深入探究创伤性脑损伤(TBI)后 T 细胞与小胶质细胞的相互作用,我们建立了体外 Transwell 共培养系统:小胶质细胞取自 CCI 小鼠损伤 24 小时后同侧大脑半球,T 细胞则来自另一组接受 CCI 造模并经鼻腔给予 aCD3 或同型对照抗体处理 7 天的小鼠脾脏(图 2)。实验中将小胶质细胞置于下室,T 细胞置于上室,培养 72 小时后对小胶质细胞进行 RT-qPCR 检测。结果显示 TBI-aCD3 组小胶质细胞的抗炎及稳态标志物(Il10、Cx3cr1 和 Cd206)表达升高,促炎标志物 Il1b 表达降低,这与我们体内实验中 TBI 后 7 天的小胶质细胞转录组数据一致。通过体外阻断 IL-10 可消除这种效应,该现象与我们体内 IL-10 中和实验的观察结果相符(图 4)。
IL-10-producing Treg cells modulate microglial phagocytosis, neuroinflammation and TBI outcomes
产生 IL-10 的调节性 T 细胞通过调控小胶质细胞吞噬功能、神经炎症反应及创伤性脑损伤预后发挥作用
FoxP3 Treg cells play a critical role in suppressing CNS inflammation in acute stroke and chronic neurological diseases87 and, as shown above, we found that adoptive transfer of FoxP3 Treg cells from nasal aCD3-treated mice ameliorated TBI and IL-10 is an important factor in this process. We then asked whether IL-10 produced by Treg cells was responsible for this effect. To address this question, we first depleted FoxP3 Treg cells using FoxP3-DTR transgenic mice that express the diphtheria toxin receptor (DTR) under control of the FoxP3 promoter and then investigated the role of lL-10-producing Treg cells in TBI by using a dual reporter system that involved the gene encoding IL-10 and transcriptomic factor FoxP3 (10BiT.FoxP3GFP)88 which allowed us to sort IL-10+ and IL-10− Treg cells. In the first FoxP3 Treg cell depletion experiment, DT was injected 3 d before CCI and repeated every 3 d until 7 d after CCI or sham operation (Fig. 7a and Extended Data Fig. 10a). We found that the depletion of FoxP3 Treg cells worsened motor function as measured by rotarod behavioral testing (Fig. 7b) and exacerbated microglial inflammation (Fig. 7c). We also observed increased expression of proinflammatory microglial markers including Il1b, Tnf, Il6, and Il18, as measured by RT–qPCR. Most importantly, we found that the depletion of FoxP3 Treg cells reduced microglial phagocytic capacity to uptake dead neurons (Fig. 7d), highlighting their pivotal role in regulating microglial phagocytosis. Based on these results, we then investigated the role of lL-10-producing Treg cells in TBI by using a dual reporter system that involved the gene encoding IL-10 and 10BiT.FoxP3GFP (ref. 88) that allowed us to sort IL-10+ and IL-10− Treg cells (Fig. 7e-f). We assessed the effect of IL-10+ and IL-10− Treg cells by adoptive transfer of these cells to FoxP3-depleted mice (Fig. 7g and Extended Data Fig. 10b). We observed that DT has similar behavior outcomes in WT TBI DT mice and the FoxP3 TBI DT-PBS group (Fig. 7h). However, we found that the adoptive transfer of IL-10+ Treg cells was associated with improvements in motor function measured by rotarod and spatial memory measured by the Y-maze compared with the mice that received IL-10− Treg cells (Fig. 7h). Moreover, IL-10+ Treg cells attenuated the microglial inflammatory response with reduction in proinflammatory markers such as Il1b, Tnf, Il6, and Il18 compared with IL-10− Treg cells (Fig. 7i). Most importantly, adoptive transfer of IL-10+ Treg cells enhanced the microglial phagocytosis of dead neurons (Fig. 7j), indicating their critical role in modulating microglial inflammation and augmenting microglial phagocytosis. Thus, IL-10 plays a critical role in the beneficial effect of nasal aCD3-induced FoxP3 Treg cells on TBI.
FoxP3 T 细胞在抑制急性脑卒中及慢性神经系统疾病的中枢神经系统炎症中起关键作用[1]。如上所述,我们发现鼻腔给予 aCD3 处理小鼠的 FoxP3 T 细胞过继转移可改善创伤性脑损伤(TBI),而 IL-10 是这一过程中的重要因子。随后我们探究了 T 细胞产生的 IL-10 是否介导了这一效应。为解决该问题,我们首先使用 FoxP3-DTR 转基因小鼠(在 FoxP3 启动子控制下表达白喉毒素受体 DTR)清除 FoxP3 T 细胞,随后通过采用同时标记 IL-10 编码基因和转录因子 FoxP3 的双报告系统(10BiT.FoxP3[6])[7],对 IL-10+和 IL-10- T 细胞进行分选,以研究产 IL-10 T 细胞在 TBI 中的作用。在首个 FoxP3 T 细胞清除实验中,于 CCI 术前 3 天注射 DT,之后每 3 天重复注射直至 CCI 或假手术后 7 天(图[12]及扩展数据图[13])。通过转棒行为学测试发现,FoxP3 T 细胞的清除会加重运动功能障碍(图[15]),并加剧小胶质细胞的炎症反应(图[16])。 我们还通过 RT-qPCR 检测发现促炎性小胶质细胞标志物 Il1b、Tnf、Il6 和 Il18 的表达增加。最重要的是,我们发现 FoxP3 T reg 细胞的耗竭会降低小胶质细胞吞噬死亡神经元的能力(图 7d ),这凸显了它们在调节小胶质细胞吞噬功能中的关键作用。基于这些结果,我们随后通过使用包含 IL-10 编码基因和 10BiT.FoxP3 GFP (参考文献 88 )的双报告系统,研究了产 IL-10 的 T reg 细胞在 TBI 中的作用,该系统使我们能够分选 IL-10 + 和 IL-10 − T reg 细胞(图 7e-f )。我们通过将这些细胞过继转移至 FoxP3 缺陷小鼠体内,评估了 IL-10 + 和 IL-10 − T reg 细胞的影响(图 7g 及扩展数据图 10b )。我们观察到 DT 在野生型 TBI DT 小鼠和 FoxP3 TBI DT-PBS 组中具有相似的行为学结果(图 7h )。然而,与接受 IL-10 − T reg 细胞的小鼠相比,过继转移 IL-10 + T reg 细胞可改善转棒测试评估的运动功能和 Y 迷宫测试评估的空间记忆(图 7h )。 此外,与 IL-10 − T reg 细胞相比,IL-10 + T reg 细胞通过降低促炎标志物(如 Il1b、Tnf、Il6 和 Il18)减轻了小胶质细胞的炎症反应(图 7i )。最重要的是,过继转移 IL-10 + T reg 细胞增强了小胶质细胞对死亡神经元的吞噬作用(图 7j ),表明其在调节小胶质细胞炎症和增强小胶质细胞吞噬功能中的关键作用。因此,IL-10 在鼻内 aCD3 诱导的 FoxP3 T reg 细胞对 TBI 的有益效应中起着至关重要的作用。
Fig. 7. IL-10-producing Treg cells play a critical role in modulating microglia and improving recovery post-TBI.
图 7. 产 IL-10 的 T reg 细胞在调节小胶质细胞和改善 TBI 后恢复中起关键作用。
a, Experimental timeline of FoxP3-DTR Treg cell depletion (created with BioRender.com). b, Behavioral testing of rotarod and Y-maze was assessed between the groups. Data are shown as mean ± s.e.m., n = 6 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. c, RT–qPCR of microglia sorted from the ipsilateral hemisphere 7 d post-TBI. Expression was normalized to GAPDH. Data are shown as mean ± s.e.m., n = 4 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. d, Phagocytosis experiment with similar design to Fig. 4b. Data are shown as box plots (min,. max., IQR, median), n = 5 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. e, Schematic representing validation of Il10 expression (created with BioRender.com). RT–qPCR of Il10 expression was done for FoxP3+Thy1.1+- and FoxP3+Thy1.1−-sorted cells and expression was normalized to GAPDH. Data are shown as mean ± s.e.m., n = 3 mice per group and analyzed by two-sided, unpaired Student’s t-test. f, Schematic representing adoptive transfer experiment (created with BioRender.com). Expression of Thy1.1 on FoxP3 was analyzed between the two groups. Data are shown as mean ± s.e.m., n = 3 sample per group and analyzed by two-sided, unpaired Student’s t-test. Each sample was a pool of five injured hemispheres. g, Schematic representing experimental timeline of adoptive transfer experiment. h, Behavioral testing of rotarod and Y-maze assessed between the groups. Data are shown as mean ± s.e.m, n = 6 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. i, RT–qPCR of microglia sorted from the ipsilateral hemisphere 7 d post-TBI. Expression was normalized to GAPDH. Data are shown as mean ± s.e.m., n = 4 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. j, Phagocytosis experiment with similar design to Fig. 4b. Data are shown as box plots (min., max., IQR, median), n = 4 mice per group and analyzed by one-way ANOVA with Tukey’s multiple comparisons. All data are biological replicates and represent two independent experiments.
a、FoxP3-DTR T 细胞清除实验时间轴(使用 BioRender.com 创建)。b、各组间进行转棒实验和 Y 迷宫行为学测试评估。数据以均值±标准误表示,每组 n=6 只小鼠,采用单因素方差分析及 Tukey 多重比较检验。c、TBI 后 7 天从同侧半球分选小胶质细胞的 RT-qPCR 结果。表达量以 GAPDH 为内参标准化。数据以均值±标准误表示,每组 n=4 只小鼠,采用单因素方差分析及 Tukey 多重比较检验。d、吞噬实验设计与图 4b 类似。数据以箱线图(最小值、最大值、四分位距、中位数)表示,每组 n=5 只小鼠,采用单因素方差分析及 Tukey 多重比较检验。e、Il10 表达验证示意图(使用 BioRender.com 创建)。对 FoxP3 + Thy1.1 + 和 FoxP3 + Thy1.1 − 分选细胞进行 Il10 表达的 RT-qPCR 检测,结果以 GAPDH 为内参标准化。数据以均值±标准误表示,每组 n=3 只小鼠,采用双侧非配对 t 检验分析。f、过继转移实验示意图(使用 BioRender.com 创建)。分析两组间 FoxP3 上 Thy1.1 的表达差异。 数据以均值±标准误表示,每组 n=3 个样本,采用双侧非配对 t 检验分析。每个样本由五个损伤半球混合组成。g 图为过继转移实验的时间流程示意图。h 图展示各组间旋转棒和 Y 迷宫行为学测试结果,数据以均值±标准误表示(每组 n=6 只小鼠),采用单因素方差分析及 Tukey 多重比较检验。i 图为 TBI 后 7 天从同侧半球分选的小胶质细胞 RT-qPCR 结果,表达量经 GAPDH 标准化,数据以均值±标准误表示(每组 n=4 只小鼠),采用单因素方差分析及 Tukey 多重比较检验。j 图吞噬实验设计与图 4b 类似,数据以箱线图(最小值、最大值、四分位距、中位数)展示(每组 n=4 只小鼠),采用单因素方差分析及 Tukey 多重比较检验。所有数据均为生物学重复,代表两次独立实验。
Extended Data Fig. 10. Validation of FoxP3 depletion in FoxP3-DTR mice following Diphtheria toxin (DT) injection and Gating strategy of FoxP3+Thy1.1+ IL-10 producing FoxP3 Tregs.
扩展数据图 10. 白喉毒素(DT)注射后 FoxP3-DTR 小鼠中 FoxP3 缺失的验证及 FoxP3 + Thy1.1 + IL-10 分泌型 FoxP3 调节性 T 细胞的门控策略
(a) Flow cytometry gating showing depletion of FoxP3+ Tregs after DT injection compared to DPBS injected for FoxP3-DTR mice 7 days post-TBI in the ipsilateral brain hemisphere, cLN, and spleen. DT was injected 3 days prior to TBI and was given every third day to sustain FoxP3 depletion. Data shown as mean ± SEM, n = 3 mice/group. Data was analyzed by two-sided unpaired Student’s t-test. The data are biological replicates and are representative from three independent experiments. (b) Gating strategy showing FoxP3+Thy1.1+ and FoxP3+Thy1.1- from the spleen/cLN of dual reporter 10BiT.FoxP3GFP mice that was selected for the adoptive transfer experiments for Fig. 7.
(a) 流式细胞术门控图显示:与 DPBS 注射组相比,FoxP3-DTR 小鼠在 TBI 后 7 天同侧大脑半球、颈淋巴结和脾脏中 FoxP3+ Treg 细胞经白喉毒素注射后的耗竭情况。白喉毒素在 TBI 前 3 天注射,并每三天给药一次以维持 FoxP3+细胞耗竭。数据显示为均值±标准误,n=3 只/组。数据采用双侧非配对 Student t 检验分析。所有数据均为生物学重复,结果来自三次独立实验。(b) 门控策略显示:来自双报告基因 10BiT.FoxP3 小鼠脾脏/颈淋巴结的 FoxP3+Thy1.1+和 FoxP3-Thy1.1+细胞群,该小鼠被选用于图 7 的过继转移实验。
Discussion 讨论
Neuroinflammation plays a crucial role in both acute and chronic stages of TBI and contributes to secondary injury11,20. Our study demonstrates that nasal aCD3-induced IL-10+FoxP3+ Treg cells promoted cognitive and motor recovery from TBI, enhanced microglia phagocytosis and reduced neuroinflammation via IL-10-dependent Treg cell–microglia crosstalk.
神经炎症在创伤性脑损伤(TBI)急慢性阶段均起关键作用,并导致继发性损伤。本研究表明,鼻腔给予抗 CD3 单抗诱导的 IL-10+FoxP3+T 细胞通过 IL-10 依赖性 T 细胞-小胶质细胞交互作用,促进 TBI 后认知与运动功能恢复,增强小胶质细胞吞噬功能并减轻神经炎症反应。
Microglia play a critical role in neuroinflammation after TBI and their activation contributes to long-term functional deficits and neurodegeneration20. We previously identified a time-dependent change in the microglial transcriptomic phenotype after contusional brain injury with reduced homeostasis, housekeeping and sensing tissue damage in the early stages and the development of a proinflammatory state over time89. Microglia also play a role in recovery by migrating to sites of neuronal death to phagocytose dead or dying cells or debris, and participate in synaptic remodeling to minimize neuronal injury and restore tissue integrity in the injured brain52. In the present study, nasal aCD3-induced FoxP3+ Treg cells enhanced the phagocytic, homeostatic, sensing and housekeeping microglial phenotype in the acute stage of injury. Transcriptomic analyses and functional studies demonstrated that nasal aCD3 increased the microglial phagocytic capacity to uptake apoptotic neurons post-injury in an IL-10-dependent manner. Importantly, we found that IL-10-producing FoxP3+ Treg cells play a critical role in augmenting microglial phagocytic capacity after TBI. We also found that aCD3 modulation of microglia at the lesion site is involved in controlling synaptic pruning and remodeling and myelin homeostasis. The expression of Bdnf, a key mediator of synaptic plasticity, which increases neuronal TrkB phosphorylation at the site of injury90, was increased in the aCD3-treated mice.
小胶质细胞在创伤性脑损伤(TBI)后的神经炎症中起关键作用,其激活会导致长期功能缺陷和神经退行性变 20 。我们先前发现挫伤性脑损伤后小胶质细胞转录组表型呈现时间依赖性变化:早期阶段组织稳态维持、管家功能和损伤感知能力下降,随时间推移逐渐发展为促炎状态 89 。同时小胶质细胞通过迁移至神经元死亡部位吞噬凋亡细胞或碎片参与修复过程,并通过突触重塑来减轻神经元损伤、恢复受损脑组织完整性 52 。本研究发现,鼻腔给予 aCD3 单抗诱导的 FoxP3 + T reg 细胞能增强急性损伤期小胶质细胞的吞噬功能,并促进其稳态维持、损伤感知及管家表型。转录组分析和功能研究表明,鼻腔 aCD3 通过 IL-10 依赖性机制提高了小胶质细胞对损伤后凋亡神经元的吞噬能力。值得注意的是,我们发现分泌 IL-10 的 FoxP3 + T reg 细胞在增强 TBI 后小胶质细胞吞噬功能中发挥关键作用。 我们还发现,aCD3 对病灶区小胶质细胞的调控参与突触修剪重塑及髓鞘稳态的调节。在 aCD3 治疗组小鼠中,突触可塑性关键介质 Bdnf 的表达量增加——该分子能促进损伤部位神经元 TrkB 磷酸化[7]。
In the chronic phase of TBI, nasal aCD3-induced FoxP3+ Treg cells regulated the function of microglia from a phagocytic phenotype in the acute stages to a homeostatic and less inflammatory phenotype. It also decreased the expression of MGnD and DAM genes, which are associated with neurodegeneration51, toward homeostatic levels seen in sham-treated animals at 30 d post-injury. By removing cellular debris by phagocytosis early after injury and releasing neurotrophic factors and anti-inflammatory cytokines, microglia contribute to the reduced cell death and improved behavioral and neuropathological outcomes observed after nasal aCD3 treatment of TBI.
在 TBI 慢性期,鼻腔给予 aCD3 诱导的 FoxP3 + T reg 细胞调控了小胶质细胞功能,使其从急性期的吞噬表型转变为稳态且炎症反应降低的表型。该治疗还使与神经退行性变相关的 MGDnD 和 DAM 基因 51 表达水平下降,在损伤后 30 天恢复至假手术组的稳态水平。通过促进小胶质细胞在损伤早期吞噬清除细胞碎片,并释放神经营养因子和抗炎细胞因子,鼻腔 aCD3 治疗改善了 TBI 后的细胞存活率,并促进了行为学表现和神经病理学转归。
Studies have shown T lymphocyte infiltration into the brain after TBI91. Treg cells comprise a population of CD4+ T cells that include FoxP3+ Treg cells and FoxP3− Treg cells, the latter of which includes TH3 and Type 1 regulatory T (Tr1) cells92. Depletion of FoxP3+ Treg cells increased T cell CNS infiltration and expression of inflammatory IFNγ after TBI27. However, the function of the interaction between Treg cells and microglia is largely unknown. Our transcriptomic analyses of peripheral and brain-infiltrating Treg cells revealed that they were reprogrammed in favor of the mobilization, immunoregulation and cell–cell interactions, especially the activation of phagocytes. Consistent with a recent study in stroke23, brain-infiltrating Treg cells exhibited higher expression of genes involved in immunoregulatory and trophic factors which were enhanced with nasal aCD3. Adoptive transfer of total CD4, CD4+FoxP3+ Treg cells and, most importantly, CD4+FoxP3+IL-10+ Treg cells from nasal aCD3-treated mice modulated the microglial response and improved behavioral and neuropathological outcomes. We reported that nasal aCD3 treatment in a progressive experimental autoimmune encephalitis (EAE) model of MS27 and a model of lupus30 induced an IL-10-dependent CD4+LAP+FoxP3+ Treg cell response. In our nasal aCD3 TBI studies, we did not observe an increase in LAP+ T cells, although we did observe expansion of CD4+FoxP3+ Treg cells and IL-10+FoxP3+ Treg cells. These differences may be related to distinct regulatory mechanisms induced by acute injury such as TBI versus an autoimmune inflammatory process such as EAE or lupus, although, in both instances, nasal aCD3 induced a Treg cell phenotype that ameliorated disease.
研究表明,创伤性脑损伤(TBI)后存在 T 淋巴细胞向脑部的浸润现象。T 细胞群体包含 CD4 阳性 T 细胞亚群,其中既有 FoxP3 阳性 T 细胞,也有 FoxP3 阴性 T 细胞,后者又包含 Tr3 细胞和 1 型调节性 T 细胞(Tr1)。当 FoxP3 阳性 T 细胞被清除后,TBI 模型中出现中枢神经系统 T 细胞浸润增加及促炎因子 IFNγ表达升高的现象。然而,T 细胞与小胶质细胞间的相互作用机制尚不明确。我们对周边及脑浸润 T 细胞的转录组分析显示,这些细胞发生了有利于细胞迁移、免疫调节及细胞间相互作用(特别是吞噬细胞激活)的重编程。与近期脑卒中研究结果一致,鼻腔给予抗 CD3 单抗可增强脑浸润 T 细胞中免疫调节因子和神经营养因子的基因表达。过继转移实验证实,来自鼻腔抗 CD3 处理小鼠的总 CD4 细胞、CD4+FoxP3+T 细胞(尤其是 CD4+FoxP3+IL-10+T 细胞)能调控小胶质细胞反应,改善行为学表现和神经病理学结局。 我们曾报道,在多发性硬化症(MS)的进展性实验性自身免疫性脑炎(EAE)模型 27 和狼疮模型 30 中,鼻腔给予 aCD3 单抗治疗可诱导 IL-10 依赖性 CD4 + LAP + FoxP3 + T reg 细胞反应。在本研究的鼻腔 aCD3 治疗创伤性脑损伤(TBI)实验中,虽未观察到 LAP + T 细胞增加,但检测到 CD4 + FoxP3 + T reg 细胞及 IL-10 + FoxP3 + T reg 细胞的扩增。这些差异可能源于 TBI 这类急性损伤与 EAE 或狼疮等自身免疫炎症过程触发的不同调控机制,但两种情况下鼻腔 aCD3 均诱导了具有疾病改善作用的 T reg 细胞表型。
Treg cells exert their immunoregulatory functions after TBI by secreting regulatory cytokines such as IL-10 (ref. 93), IL-4 and TGF-β94,95. IL-10 is an anti-inflammatory cytokine that plays a role in resolution of neuroinflammation after brain injury93. The expression of IL-10 in the brain increases with acute brain injury, promoting neurological recovery by multiple mechanisms, including inhibiting microglia or macrophage cytokine production, reducing the activation of effector T cells, monocytes and macrophages, and promoting neuronal and glial cell survival93,96,97. Clinical studies have shown that IL-10 levels increase after TBI98,99. Preclinical studies have shown that IL-10−/− female mice have worse inflammation and motor and cognitive function post-CCI100. IL-10 pharmacological formulations reduce lesion volume and improve functional outcomes after TBI101,102. However, the role of IL-10 in Treg cell–microglia crosstalk and its effects on microglial function after TBI have not been explored. We found that nasal aCD3 mAb induced IL-10-producing FoxP3+ Treg cells that migrated to the CNS to downregulate microglial activation and improve behavior in an IL-10-dependent manner. Specific blocking of the IL-10R in vivo in microglia reversed the therapeutic effects of nasal aCD3 mAb, reduced microglial phagocytic capacity and contributed to a chronic microglial activation state. These results demonstrate the important role of Treg cell–microglial crosstalk via IL-10 in the treatment of TBI using nasal aCD3.
T 细胞通过分泌 IL-10(参考文献 93 )、IL-4 和 TGF-β(参考文献 94,95 )等调节性细胞因子,在创伤性脑损伤(TBI)后发挥免疫调节功能。IL-10 作为一种抗炎细胞因子,在脑损伤后神经炎症的消退过程中起重要作用(参考文献 93 )。急性脑损伤时脑内 IL-10 表达增加,通过多种机制促进神经功能恢复,包括抑制小胶质细胞或巨噬细胞因子产生、减少效应 T 细胞/单核细胞/巨噬细胞的活化,以及促进神经元和胶质细胞存活(参考文献 93,96,97 )。临床研究表明 TBI 后 IL-10 水平会升高(参考文献 98,99 )。临床前研究显示 IL-10 缺陷(参考文献 −/− )雌性小鼠在控制性皮质撞击(CCI)后会出现更严重的炎症反应及运动认知功能障碍(参考文献 100 )。IL-10 药物制剂可减小 TBI 后病灶体积并改善功能预后(参考文献 101,102 )。然而 IL-10 在 T 细胞-小胶质细胞互作中的作用及其对 TBI 后小胶质细胞功能的影响尚未被阐明。 我们发现鼻内给予 aCD3 单抗可诱导产生 IL-10 的 FoxP3 + T reg 细胞,这些细胞迁移至中枢神经系统,通过 IL-10 依赖性途径下调小胶质细胞活化并改善行为学表现。特异性阻断小胶质细胞中的 IL-10 受体可逆转鼻内 aCD3 单抗的治疗效果,降低小胶质细胞的吞噬能力,并导致慢性小胶质细胞活化状态。这些结果证实了 T reg 细胞-小胶质细胞通过 IL-10 进行交互在鼻内 aCD3 治疗创伤性脑损伤中的重要作用。
A major challenge for the treatment of TBI is to induce Treg cells in a fashion that is nontoxic and translatable to the clinic. Approaches such as infusion of cord blood Treg cells, astrocytes engineered to produce IL-2 and mesenchymal stromal cells have demonstrated preclinical benefit, but are difficult to translate to the clinic87,103. Nasal aCD3 is a unique immunotherapeutic approach to induce Treg cells to downregulate CNS inflammation and could be given immediately after TBI. Foralumab, a fully human aCD3 mAb, has been successfully given to humans and has demonstrated immunomodulatory effects with minimal toxicity104–106. A pilot trial in individuals with mild-to-moderate COVID-19 reduced lung inflammation and blood inflammatory biomarkers without side effects105,106 and nasal foralumab is being studied in individuals with progressive MS.
创伤性脑损伤(TBI)治疗面临的主要挑战在于如何以无毒且可临床转化的方式诱导 T reg 细胞。诸如输注脐带血 T reg 细胞、经改造可产生 IL-2 的星形胶质细胞以及间充质基质细胞等方法虽在临床前研究中显示获益,但难以转化为临床应用 87,103 。鼻腔给药抗 CD3 单抗(aCD3)是一种独特的免疫治疗策略,可通过诱导 T reg 细胞来下调中枢神经系统炎症反应,且可在 TBI 发生后立即使用。全人源化抗 CD3 单抗 Foralumab 已成功应用于人体,并展现出免疫调节作用且毒性极低 104–106 。一项针对轻中度 COVID-19 患者的先导性试验显示,该疗法可减轻肺部炎症并降低血液炎症生物标志物水平,且未出现副作用 105,106 ;目前鼻腔给药的 foralumab 正在进展型多发性硬化症患者中进行研究。
In conclusion, our study identifies nasal aCD3 as a new therapeutic approach for treating TBI. Nasal aCD3 induces Treg cells that enhance the phagocytic capacity of microglia to clear dead neurons, modulate the CNS innate and adaptive immune responses, and promote functional recovery in an IL-10-dependent manner. This approach has potential applications for the treatment of TBI and other types of acute brain injury, such as stroke.
总之,我们的研究发现鼻腔给予 aCD3 单抗是治疗创伤性脑损伤(TBI)的新疗法。鼻腔 aCD3 能诱导 T reg 细胞增强小胶质细胞的吞噬能力以清除死亡神经元,调节中枢神经系统先天性和适应性免疫反应,并通过 IL-10 依赖性机制促进功能恢复。该方法在 TBI 及其他急性脑损伤(如脑卒中)治疗中具有潜在应用价值。
Methods 方法
Experimental animals 实验动物
Studies were performed using 8- to 12-week-old C57BL6J mice (Jackson Laboratories cat. no. 000664), B6.CD45.1 mice (Jackson Laboratory, cat. no. 002014), FoxP3-GFP mice (Jackson Laboratory, cat. no. 023800), FoxP3-DTR mice (Jackson Laboratory, cat. no. 016958), IL-10 KO mice (Jackson Laboratory, cat. no. 002251), Tmem119-CreETR2 mice (Jackson Laboratory, cat. no. 031820), IL-10raFlx mice (Jackson Laboratory, cat. no. 028146) and 10BiT.FoxP3GFP (kindly provided by V. Kuchroo)88. All mice were housed under specific pathogen-free conditions, with free access to food and water. All animals were housed in temperature (20 °C) and humidity (60%)-controlled rooms, maintained on a 12 h:12 h light:dark cycle (lights on at 07:00). Mice were euthanized by CO2 inhalation. The Institutional Animal Care and Use Committee at Harvard Medical School and Brigham and Women’s Hospital has all experimental procedures involving animals.
实验采用 8 至 12 周龄的 C57BL6J 小鼠(杰克逊实验室,货号 000664)、B6.CD45.1 小鼠(货号 002014)、FoxP3-GFP 小鼠(货号 023800)、FoxP3-DTR 小鼠(货号 016958)、IL-10 基因敲除小鼠(货号 002251)、Tmem119-Cre ETR2 小鼠(货号 031820)、IL-10ra Flx 小鼠(货号 028146)及 10BiT.FoxP3 GFP 小鼠(由 V. Kuchroo 教授惠赠) 88 。所有小鼠均在无特定病原体条件下饲养,自由摄食饮水。动物房环境参数为恒温 20℃、相对湿度 60%,光/暗周期 12 小时:12 小时(07:00 开灯)。采用二氧化碳 2 吸入法实施安乐死。哈佛医学院及布莱根妇女医院的机构动物护理与使用委员会批准了所有动物实验方案。
Treatment with aCD3 mAb
抗 CD3 单克隆抗体治疗
Mice were nasally treated with a daily dose, immediately (4–6 hours) and for some experiments early (3 days) or delayed (7 days) post TBI, of 1 µg per mouse hamster immunoglobulin G (IgG) CD3-specific antibody (BioXCell, clone no. 145-2C11) or hamster IgG control antibody (BioXCell) dissolved in PBS and henceforth every other day after the first week until the experimental endpoint. For some experiments, mice were given 0.5 mg of monoclonal anti-IL-10R-blocking antibody (BioXCell, clone 1B1.3A), by intraperitoneal injection at the onset of TBI and henceforth every third day until the experimental endpoint.
小鼠经鼻给予每日剂量处理,在创伤性脑损伤(TBI)后立即(4-6 小时)进行,部分实验采用早期(3 天)或延迟(7 天)给药方案,每只小鼠使用 1μg 仓鼠免疫球蛋白 G(IgG)CD3 特异性抗体(BioXCell,克隆号 145-2C11)或溶解于 PBS 的仓鼠 IgG 对照抗体(BioXCell),首周后改为隔日给药直至实验终点。部分实验中,小鼠在 TBI 发生时通过腹腔注射给予 0.5mg 单克隆抗 IL-10R 阻断抗体(BioXCell,克隆 1B1.3A),之后每三日给药一次直至实验终点。
Conditional genetic deletion of IL-10Ra in microglia
小胶质细胞中 IL-10Ra 的条件性基因敲除
To induce Cre-recombinase expression, a dose of tamoxifen (150 mg per kg of body weight) in corn oil was injected i.p. for 5 d consecutively. IL-10Raflx/flx was crossed with Tmem119-CreETR2 mice110. Recombination was induced in Tmem119-CreETR2:IL-10Raflx/flx mice and Tmem119-CreWT:IL-10Raflx/flx littermates were used as controls. A washout period of 2 weeks was implemented after the last tamoxifen injection before starting any experiment.
为诱导 Cre 重组酶表达,连续 5 天腹腔注射玉米油溶解的他莫昔芬(150 mg/kg 体重)。将 IL-10Ra flx/flx 小鼠与 Tmem119-Cre ETR2 小鼠 110 杂交。在 Tmem119-Cre ETR2 :IL-10Ra flx/flx 小鼠中诱导重组,并以 Tmem119-Cre WT :IL-10Ra flx/flx 同窝仔作为对照。末次他莫昔芬注射后实施 2 周洗脱期方可开始实验。
FoxP3 cell depletion FoxP3 细胞耗竭
Depletion of FoxP3 was done as previously described23. In short, DT (0.05 μg per g body weight) was injected i.p. 3 d before TBI and was repeated every 3 d to maintain FoxP3 depletion until sacrifice.
FoxP3 耗竭操作如前所述 23 。简言之,在创伤性脑损伤(TBI)前 3 天腹腔注射 DT(0.05 μg/g 体重),之后每 3 天重复注射一次以维持 FoxP3 耗竭状态直至处死。
Controlled cortical impact
控制性皮质撞击
A CCI model was used as previously described111. Mice were anesthetized with 4.5% isoflurane (Anaquest) in 70% nitrous oxide and 30% oxygen using a Fluotec 3 vaporizer (Colonial Medical). The mice were placed in a stereotaxic frame and a 5-mm craniotomy was made over the right somatosensory cortex using a drill and a trephine. The bone flap was removed and discarded and a pneumatic cylinder with a 1.5- or 3-mm flat tip impounder and velocity 6 m s−1, depth 1.0 or 1.5 mm and dwell time of 0.8 s was used to induce CCI (Impact One, Leica Biosystems). The scalp was sutured closed and the mice were returned to their cages to recover.
采用先前描述的 CCI 模型 111 。使用 Fluotec 3 蒸发器(Colonial Medical)以 4.5%异氟烷(Anaquest)与 70%一氧化二氮和 30%氧气的混合气体麻醉小鼠。将小鼠固定于立体定位仪上,使用钻头和环钻在右侧体感皮层区域制作 5 毫米颅骨开窗。移除骨瓣后,采用配备 1.5 或 3 毫米平头撞击器、速度 6 米/秒 −1 、深度 1.0 或 1.5 毫米、停留时间 0.8 秒的气动撞击装置(Impact One,Leica Biosystems)诱导 CCI 损伤。缝合头皮后将小鼠放回笼中恢复。
Behavioral studies 行为学研究
Open field testing 旷场实验
The open field (OF) test is used to measure general locomotor activity and anxiety-like behavior of the animals112. The OF square chambers are made of blue Plexiglas with dimensions of 30 × 38 × 40 cm3. For each testing session, the animal was allowed to explore the chamber for 15 min. A computer-assisted tracking system and software (EthoVision XT, v.14, Noldus Information Technology) was used to record the behavior of the animals throughout the testing session. The percentage time spent in the center was measured.
旷场实验(OF)用于评估动物的基础运动活性和焦虑样行为 112 。实验装置为蓝色有机玻璃制成的方形箱体(30×38×40 cm) 3 。每次测试时,动物可在箱体内自由探索 15 分钟。采用计算机辅助追踪系统及软件(EthoVision XT v.14,Noldus Information Technology)全程记录动物行为,重点测量动物在中央区域停留时间的百分比。
Rotarod 转棒测试
The rotarod was carried out as previously described31. Mice were placed on a rotarod apparatus (Ugo Basile, cat. no. 7650), accelerating from 4 rpm to 60 rpm in 300 s. Each animal was given three trials and the times when the animal would no longer be able to hold on were recorded and averaged for analysis of motor function.
转棒测试方法参照既往文献 31 。将小鼠置于转棒仪(Ugo Basile,货号 7650)上,转速在 300 秒内从 4 转/分钟加速至 60 转/分钟。每只动物进行 3 次测试,记录其从转棒掉落的时间并取平均值用于运动功能分析。
Morris water maze 莫里斯水迷宫
The MWM was used to measure spatial learning and memory by training mice to use spatial cues to find a hidden platform to escape water113. The MWM apparatus is a circular pool with a diameter of 130 cm and depth of 50 cm. During the first day, the platform was visible and the animals were given three trials to find it. During the 4-d training period, mice received three trials per day learning how to find the hidden platform. Then 24 h after the last training day, a probe trial was performed in which the platform was removed and mice were allowed to swim for up to 60 s. The amount of time spent by the animal to find the platform and the time spent in the target quadrant for the probe trial were calculated using Noldus EthoVision XT tracking software.
采用莫里斯水迷宫(MWM)评估空间学习和记忆能力,通过训练小鼠利用空间线索寻找隐藏平台以逃离水域 113 。该迷宫装置为直径 130 厘米、深 50 厘米的圆形水池。首日训练中平台可见,动物需完成三次定位测试。在为期 4 天的训练期内,小鼠每天接受三次寻找隐藏平台的训练。末次训练 24 小时后进行探测试验,此时移除平台并允许小鼠自由游泳 60 秒。使用 Noldus EthoVision XT 追踪软件计算动物找到平台所需时间及探测试验中在目标象限停留时长。
Y-maze Y 型迷宫
The Y-maze was used to assess spatial working memory in mice. The test was conducted as previously described114. In short, the mice were placed in the center of the maze and given 5 min to explore all three arms, the number of triads (triplets of consecutive arm entries of ABC, BCA and CAB) were counted to the percentage alternation. The ratio of correct choice was determined by the equation: Percentage alternations = ((No. of alternations)/(Total arm entries − 2)) × 100 (ref. 115).
采用 Y 迷宫评估小鼠的空间工作记忆。测试方法如前所述 114 。简言之,将小鼠置于迷宫中央,给予 5 分钟时间探索所有三个臂区,记录 ABC、BCA 和 CAB 三种连续臂区进入组合(即三连交替)的次数以计算交替百分比。正确选择率通过公式确定:交替百分比=(交替次数/(总臂区进入次数−2))×100(参考文献 115 )。
BBB permeability 70-kDa FITC-dextran
血脑屏障通透性检测用 70-kDa FITC-葡聚糖
A 70-kDa FITC-dextran (Sigma-Aldrich, cat. no. 46945) was used to measure BBB permeability as described in previous studies116,117, with modifications: 0.2 mg of 70-kDa FITC-dextran per g of body weight was injected retro-orbitally118,119. Then, 10 min post-70-kDa FITC-dextran administration, the mouse was euthanized with a lethal dose of xylazine and ketamine cocktail (450 mg kg−1 and 45 mg kg−1) i.p. with a 29-gauge insulin pen. The mouse was transcardially perfused with 50 ml of PBS. The whole brain was removed and stored at −80 °C (ref. 120). Cryoprotected and flash-frozen brains were coronally sectioned (16-μm-thick serial sections, 300 μm apart)116. The brain sections were captured by a Leica DMi8 wide-field microscope. FITC-dextran visualization was done under a 488-nm excitation wavelength laser. For each mouse, we obtained five to six continued section slices from the brain tissue’s front, middle and posterior sections. An investigator blind to the experimental design manually measured the l mean gray value of the dextran tracer-positive area found in the brain parenchyma using ImageJ.
采用 70-kDa FITC-葡聚糖(Sigma-Aldrich,货号 46945)检测血脑屏障通透性,方法参照前期研究 116,117 并稍作改良:按每克体重 0.2 mg 剂量经眶后静脉注射 118,119 。注射 10 分钟后,使用 29G 胰岛素笔经腹腔注射致死剂量的赛拉嗪-氯胺酮混合液(450 mg/kg −1 和 45 mg/kg −1 )处死小鼠。经心脏灌注 50 ml PBS 后取出全脑,保存于-80°C(参考文献 120 )。经冷冻保护处理的速冻脑组织进行冠状切片(连续 16μm 厚切片,间隔 300μm) 116 ,使用 Leica DMi8 宽场显微镜采集脑切片图像,在 488 nm 激发波长激光下观察 FITC-葡聚糖信号。每只小鼠取脑组织前、中、后部 5-6 张连续切片,由不知实验分组的研究人员采用 ImageJ 软件手动测量脑实质内葡聚糖示踪剂阳性区域的平均灰度值。
Brain edema 脑水肿检测
Brains were removed at 72 h after CCI, bisected into left and right hemispheres and each hemisphere was weighed (wet weight). Brains were then dried at 60 °C for 48 h and dry weights were obtained. The percentage of brain water content was expressed as ((wet − dry weight)/(wet weight)) × 100% as previously described121.
在 CCI 后 72 小时取出脑组织,将其分为左右半球并分别称重(湿重)。随后将脑组织置于 60°C 环境下干燥 48 小时以获得干重。按先前描述的方法 121 ,脑含水量百分比计算公式为:((湿重-干重)/湿重)×100%。
Magnetic resonance imaging
磁共振成像
Imaging was done using a 7.0T Bruker BioSpect USR. In brief, mice were gently handled and placed in an isoflurane anesthesia chamber. Then the mice were placed inside the imaging apparatus with their nose in front of tubes releasing 2% isoflurane. Electrocardiogram (ECG) leads were placed on the animal’s paws and a pneumatic pillow sensor placed under the abdomen for continuous ECG and respiratory rate monitoring of the anesthetized animal. These waveforms were closely monitored throughout magnetic resonance imaging (MRI) by the MRI operator. The animal was placed on an MRI-compatible bed, which was placed inside the magnet for imaging. The imaging sessions lasted between 15 min and 60 min. Mice were then returned to their cages and monitored continuously after being returned to their cages before returning to a fully alert status. The following parameters were obtained to generate the T2 sequence images: slice thickness: 0.5 mm; repetition time: 3,000 ms; echo time: 50 ms; no. of averages: 3; spacing between slices: 0.5 mm; echo train length: 8; acquisition matrix: 200 × 200; flip angle: 90°; and field of view: 20 mm. Serial Images were viewed and analyzed using the three-dimensional (3D) Slicer platform122.
采用 7.0T 布鲁克 BioSpect USR 进行影像采集。简要流程如下:轻柔固定小鼠后置于异氟烷麻醉舱,随后将其鼻部对准输送 2%异氟烷的导管安置于成像设备内。动物四肢连接心电图(ECG)导联,腹部下方放置气垫式呼吸传感器,持续监测麻醉状态下的心电及呼吸频率。磁共振成像(MRI)过程中由操作人员全程监控这些波形参数。实验动物被安置于磁共振兼容扫描床上,推入磁体进行成像,单次扫描时长 15-60 分钟。扫描完成后将小鼠放回笼具持续观察,直至完全恢复清醒状态。获取 T2 序列图像的参数设置为:层厚 0.5mm;重复时间 3000ms;回波时间 50ms;平均次数 3 次;层间距 0.5mm;回波链长度 8;采集矩阵 200×200;翻转角 90°;视野 20mm。 使用三维(3D)Slicer 平台对连续图像进行查看和分析 122 。
Immunohistochemistry 免疫组织化学
Animals were anesthetized with CO2 until the respiration rate slowed and perfused transcardially with Hanks’ balanced salt solution (HBSS). Brains were post-fixed in 4% paraformaldehyde for 48 h, then transferred to a 15% sucrose solution for 24 h and finally transferred to a 30% sucrose solution for 24 h. Brains were then flash frozen in Tissue-Tek Oct (Sakura, compound 4583) and stored at −80 °C until the time of sectioning. Brains were subsequently sectioned at −20 °C using a cryostat at the bregma position for each targeted brain. Sections were cut at 0.2 mm in a fourfold series interval. Five total sections were placed on Colorfrost Plus-treated adhesion slides (Thermo Fisher Scientific) and stored at −20 °C until the time of staining. Whole meninges were processed as previously described123. For immunofluorescence, sections were blocked in in a 10% normal horse serum solution, containing 0.1% Triton X-100, 1% glycine and 2% bovine serum albumin (BSA). Slides were incubated overnight at 4 °C with anti-Iba-1 (rabbit, 1:1,000, Wako). The following day sections were washed and incubated with an Alexa Fluor-647 goat anti-rabbit IgG (1:1,000, Abcam, cat. no. ab150075) for 1 h at room temperature. Sections were also stained with H&E (Abcam, cat. no. ab245880) and TUNEL (TUNEL Assay Kit, BrdU-Red, Abcam, cat. no. ab66110) according to their corresponding kit protocols. Iba-1- and TUNEL-stained slides were co-stained with DAPI mounting medium (Vector Laboratories, cat. no. UX-93952-24). FoxP3 GFP mice were used to visualize FoxP3 Treg cells in the meninges and brain using the Cy2 channel of the microscope and CD3 was stained using Alexa Fluor-647 anti-mouse CD3 (BioLegend, cat. no. 17A2, 1:50). Images were taken using a Leica DMi8 wide-field microscope on the ×20 objective or the Zeiss LSM710 confocal microscope.
实验动物采用 CO 2 麻醉至呼吸频率减缓后,经心脏灌注汉克斯平衡盐溶液(HBSS)。脑组织在 4%多聚甲醛中后固定 48 小时,随后转入 15%蔗糖溶液 24 小时,最终转移至 30%蔗糖溶液 24 小时。脑组织随后用 Tissue-Tek OCT 包埋剂(Sakura,货号 4583)快速冷冻,保存于-80°C 直至切片。在-20°C 条件下使用冷冻切片机沿前囟定位进行脑组织切片,以 0.2mm 厚度进行四倍连续切片。将五组切片置于 Colorfrost Plus 处理过的防脱载玻片(赛默飞世尔科技)上,-20°C 保存待染色。脑膜处理参照先前方法 123 。免疫荧光实验中,切片用含 0.1% Triton X-100、1%甘氨酸和 2%牛血清白蛋白(BSA)的 10%正常马血清封闭液处理,4°C 条件下与抗 Iba-1 抗体(兔源,1:1000,和光纯药)孵育过夜。次日洗涤后与 Alexa Fluor-647 标记的山羊抗兔 IgG 二抗(1:1000,艾博抗,货号 室温下使用抗 CD3 单克隆抗体(Abcam,货号 ab150075)孵育 1 小时。切片还根据相应试剂盒说明书进行了 H&E 染色(Abcam,货号 ab245880)和 TUNEL 染色(TUNEL 检测试剂盒,BrdU-红色,Abcam,货号 ab66110)。Iba-1 和 TUNEL 染色切片使用 DAPI 封片剂(Vector Laboratories,货号 UX-93952-24)进行共染。采用 FoxP3 GFP 小鼠通过显微镜 Cy2 通道观察脑膜和脑组织中 FoxP3 T reg 细胞,并使用 Alexa Fluor-647 标记的小鼠抗 CD3 抗体(BioLegend,货号 17A2,1:50 稀释)进行 CD3 染色。图像采集使用 Leica DMi8 宽场显微镜(20 倍物镜)或 Zeiss LSM710 共聚焦显微镜完成。
Image analysis 图像分析
Analysis of the percentage Iba-1 and number of TUNEL-positive cells per surface area was performed on five photomicrographs per animal (n = 4 or 5). The sections analyzed were taken between 300 μm and 1,500 μm laterally from the coronal plane. Each scanned photomicrograph was used to produce images of the area of contusion. All the images were analyzed using ImageJ software (National Institutes of Health: https://imagej.nih.gov/ij). Images were split by color channel, the channel of interest was threshold using the Yen setting and the number of positive cells were quantified as previously described124.
对每只动物(n=4 或 5)的五张显微照片进行 Iba-1 阳性率及单位面积 TUNEL 阳性细胞数分析。所选切片位于冠状面外侧 300μm 至 1500μm 区间。每张扫描显微照片均用于生成挫伤区域图像,所有图像均采用 ImageJ 软件(美国国立卫生研究院: https://imagej.nih.gov/ij )进行分析。图像经颜色通道分离后,使用 Yen 设定对目标通道进行阈值处理,阳性细胞计数方法如文献 124 所述。
Serum biomarkers 血清生物标志物检测
Levels of cytokines in the serum of mice were measured with the V-PLEX Plus Proinflammatory Panel1 Mouse Kit (Meso Scale Discovery, cat. no. K15048G-1). All sample controls were diluted 1:2 and run as duplicates according to the manufacturer’s protocol, as previously described125.
采用 V-PLEX Plus 促炎因子 Panel1 小鼠检测试剂盒(Meso Scale Discovery,货号 K15048G-1)测定小鼠血清细胞因子水平。所有样本按 1:2 稀释,依照制造商方案进行双孔检测,具体方法参见文献 125 。
Quanterix single-molecule array analysis
Quanterix 单分子阵列分析
Levels of ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) and GFAP were quantified in the serum using single-molecule array technology (SiMoA, Quanterix). The SiMoA Neurology 4-Plex B kit was run according to the manufacturer’s directions. Briefly, samples were thawed, vortexed and centrifuged at 10,000g for 5 min. All samples were run at a 4× dilution, along with eight calibrators run neat and two controls also run at a 4× dilution. The data were validated using a calibration curve with R2 > 0.99. For both UCHL-1 and GFAP, the dynamic range was 0–40,000 pg ml−1, with a lower limit of quantification of 9.38 pg ml−1.
采用单分子阵列技术(SiMoA,Quanterix)定量检测血清中泛素羧基末端水解酶 L1(UCH-L1)和 GFAP 水平。严格按制造商说明操作 SiMoA Neurology 4-Plex B 试剂盒:样本解冻后涡旋震荡,10,000g 离心 5 分钟。所有样本按 4 倍稀释度检测,同时运行 8 个未稀释校准品和 2 个 4 倍稀释对照品。通过 R²>0.99 的校准曲线验证数据。UCHL-1 和 GFAP 的检测动态范围均为 0-40,000 pg/ml,定量下限为 9.38 pg/ml。
Flow cytometry microglial sorting
流式细胞术小胶质细胞分选
For microglial cell sorting, mice were anesthetized with CO2 until their respiration rate slowed and then transcardially perfused with 50 ml of HBSS containing heparin (1:1,000). After perfusion, the ipsilateral hemisphere was homogenized using a Dounce glass tissue homogenizer. Cells were separated through a Percoll (GE Healthcare Life Sciences) 30% gradient centrifugation. Cells were isolated from the Percoll layer and stained on ice for 30 min with combinations of phycoerythrin (PE)/cyanine7 anti-mouse CD11b (BioLegend, cat. no. M1/70, 1:100), allophycocyanin (APC)/cyanine7 anti-mouse CD45 (BioLegend, cat. no. 30-F11, 1:100), FITC anti-mouse Ly6C (BD Bioscience, cat. no. AL-21, 1:200) and APC anti-mouse 4D4 (ref. 51) (marking resident microglia; 1:1,000) in blocking buffer containing 0.2% BSA (Sigma-Aldrich) in HBSS. Cell sorting was performed using a FACSAriaIII cell sorter (Becton Dickson). Microglial cells were identified as CD45+CD11b+Ly6C−4D4+ and dead cells were also excluded based on 7-aminoactinomycin D (7-AAD; BD Bioscience) staining. Cells were sorted directly in 1.5-ml Eppendorf tubes and stored at −80 °C. Phagocytic positive microglia were sorted as 4D4+ Alexa-405+.
为进行小胶质细胞分选,实验小鼠经 CO 2 麻醉至呼吸频率减缓后,经心脏灌注 50ml 含肝素(1:1000)的 HBSS 溶液。灌注完成后,使用 Dounce 玻璃组织匀浆器对同侧大脑半球进行匀浆处理。细胞通过 30% Percoll(GE Healthcare Life Sciences)梯度离心分离。从 Percoll 层分离出的细胞在冰上用以下抗体组合染色 30 分钟:藻红蛋白(PE)/花青素 7 标记的抗小鼠 CD11b(BioLegend,货号 M1/70,1:100)、别藻蓝蛋白(APC)/花青素 7 标记的抗小鼠 CD45(BioLegend,货号 30-F11,1:100)、FITC 标记的抗小鼠 Ly6C(BD Bioscience,货号 AL-21,1:200)以及 APC 标记的抗小鼠 4D4(参考文献 51 )(标记定居型小胶质细胞;1:1000),所用封闭缓冲液为含 0.2% BSA(Sigma-Aldrich)的 HBSS 溶液。细胞分选采用 FACSAriaIII 流式细胞分选仪(Becton Dickson)完成。小胶质细胞鉴定标准为 CD45 + CD11b + Ly6C − 4D4 + ,同时通过 7-氨基放线菌素 D(7-AAD;BD Bioscience)染色排除死细胞。分选后的细胞直接收集于 1.5ml EP 管中,保存于-80°C 环境。 吞噬活性阳性小胶质细胞被分选标记为 4D4 + Alexa-405 + 。
Flow cytometry intracellular staining
流式细胞术细胞内染色
Intracellular cytokine staining and cell isolation were done as previously described126. The meninges were carefully removed from the skull and the ipsilateral brain was further isolated. The enzyme dissociation mix used for the ipsilateral brain hemisphere and meninges was collagenase P (0.5 mg ml−1; Sigma-Aldrich, cat. no. 11213865001) and DNase-1 (250 U ml−1, Worthington, cat. no. LK003172) diluted in Roswell Park Memorial Institute (RPMI)-1640 with 10 mM Hepes. The samples were then finely minced and incubated in a room temperature shaker for 1 h. After enzyme dissociation, the cells were separated using Percoll (GE Healthcare Life Sciences) as described above. Cells isolated from the brain and meninges were incubated for only 2 h instead of the 4 h for cLN cells. Acquisition was performed on a Symphony (BD Biosciences) using DIVA software (BD Biosciences) and the data were analyzed with FlowJo software v.9.9 or v.10.1 (TreeStar Inc.). Intracellular staining antibodies used Zombie Aqua Fixable Viability Kit (BioLegend, cat. no. 423102, 1:1,000) or Zombie UV (BioLegend, cat. no. 423108, 1:1,000) was used to exclude dead cells. The staining antibodies used PE/cyanine7 anti-mouse CD11b (BioLegend, cat. no. M1/70, 1:300), APC/cyanine7 anti-mouse TCR-β (BioLegend, cat. no. H57-597, 1:100), BUV661 anti-mouse CD45 (30-F11, BD Biosciences, 1:200), PE anti-mouse CD4 (BioLegend, cat. no. GK1.5, 1:100), BUV805 anti-mouse CD8 (BD Biosciences, cat. no. 53-6.7, 1:100), FITC anti-mouse FoxP3 (eBioscience, cat. no. FJK-16s, 1:100), BV421 anti-mouse LAP (BioLegend, cat. no. TW7-16B4, 1:100), PE/Dazzle 594 anti-mouse IL-10 (BioLegend, cat. no. JES5-16E3, 1:100), BUV395 anti-mouse IL-17a (BD Biosciences, cat. no. TC11-18H10, 1:100), BV785 anti-mouse IFNγ (BioLegend, cat. no. XMG1.2, 1:100), BV605 anti-mouse Ly6G (BioLegend, cat. no. 1A8, 1:300), BV711 anti-mouse NK-1.1 (BioLegend, cat. no. PK136, 1:100), AF700 anti-mouse Ly6C (BioLegend, cat. no. HK1.4, 1:200), BV711 anti-mouse Ly6C (BioLegend, cat. no. HK1.4, 1:200) and APC anti-mouse 4D4 (ref. 47) (1:1,000) provided by Butovsky.
细胞内细胞因子染色及细胞分离操作参照先前文献方法 126 。小心剥离颅骨脑膜后,进一步分离同侧脑组织。用于同侧大脑半球及脑膜酶解混合液成分为:胶原酶 P(0.5 mg/ml −1 ;Sigma-Aldrich 货号 11213865001)与 DNase-1(250 U/ml −1 ,Worthington 货号 LK003172 )溶于含 10 mM Hepes 的 RPMI-1640 培养基。组织样本精细剪碎后置于室温摇床孵育 1 小时。酶解完成后采用前述 Percoll(GE Healthcare Life Sciences)梯度离心法分离细胞。脑组织及脑膜来源细胞仅孵育 2 小时(对比 cLN 细胞的 4 小时)。使用 Symphony 流式细胞仪(BD Biosciences)配合 DIVA 软件(BD Biosciences)进行数据采集,FlowJo 软件 v.9.9 或 v.10.1(TreeStar Inc.)分析数据。细胞内染色采用 Zombie Aqua 可固定活力检测试剂盒(BioLegend 货号 423102,1:1000)或 Zombie UV(BioLegend 货号 423108,1:1000)排除死细胞。 实验所用染色抗体包括:PE/cyanine7 标记的小鼠 CD11b 抗体(BioLegend,货号 M1/70,1:300)、APC/cyanine7 标记的小鼠 TCR-β抗体(BioLegend,货号 H57-597,1:100)、BUV661 标记的小鼠 CD45 抗体(30-F11,BD Biosciences,1:200)、PE 标记的小鼠 CD4 抗体(BioLegend,货号 GK1.5,1:100)、BUV805 标记的小鼠 CD8 抗体(BD Biosciences,货号 53-6.7,1:100)、FITC 标记的小鼠 FoxP3 抗体(eBioscience,货号 FJK-16s,1:100)、BV421 标记的小鼠 LAP 抗体(BioLegend,货号 TW7-16B4,1:100)、PE/Dazzle 594 标记的小鼠 IL-10 抗体(BioLegend,货号 JES5-16E3,1:100)、BUV395 标记的小鼠 IL-17a 抗体(BD Biosciences,货号 TC11-18H10,1:100)、BV785 标记的小鼠 IFNγ抗体(BioLegend,货号 XMG1.2,1:100)、BV605 标记的小鼠 Ly6G 抗体(BioLegend,货号 1A8,1:300)、BV711 标记的小鼠 NK-1.1 抗体(BioLegend,货号 PK136,1:100)、AF700 标记的小鼠 Ly6C 抗体(BioLegend,货号 HK1.4,1:200)、BV711 标记的小鼠 Ly6C 抗体(BioLegend,货号 HK1.4,1:200)以及 Butovsky 提供的 APC 标记的小鼠 4D4 抗体(文献编号 47 ,1:1,000)。
Flow cytometry FoxP3(GFP) Treg cell sorting from the brain and blood
流式细胞术从脑组织和血液中分选 FoxP3(GFP) T reg 细胞
The ipsilateral brain hemisphere was processed with the same enzyme dissociation kit described above and then processed as the microglial cell sorting above. The sample was purified and enriched using CD4 cell isolation microbead kit (Miltenyi Biotech, cat. no. 130-104-454) on a magnetic MACS separator before sorting. Cell sorting was performed using FACSAriaIII cell sorter (Becton Dickson). APC/cyanine7 anti-mouse CD45 (BioLegend, cat. no. 30-F11, 1:100), APC anti-mouse CD4 antibody (BioLegend, cat. no. GK1.5, 1:100) and 7-AAD (BD Bioscience) was used to identify the live CD4+ population and the FITC channel was used to identify the FoxP3+ population. Each RNA-seq sample is a pool of 5 ipsilateral hemispheres and 20 sham brain hemispheres. The blood samples were collected in heparin-coated tubes and then transferred to 15-ml tubes where ACK lysis buffer was used to lyse the erythrocytes. The sample was then strained through a 70-μm filter and stained as decribed above. Each RNA-seq sample is a pool of five mice.
同侧大脑半球采用上述相同的酶解试剂盒处理,随后按照小胶质细胞分选流程进行操作。样本在分选前使用 CD4 细胞分离磁珠试剂盒(Miltenyi Biotech,货号 130-104-454)通过 MACS 磁珠分选仪进行纯化富集。细胞分选采用 FACSAriaIII 流式细胞仪(Becton Dickson)完成,使用 APC/cyanine7 标记的小鼠 CD45 抗体(BioLegend,货号 30-F11,1:100)、APC 标记的小鼠 CD4 抗体(BioLegend,货号 GK1.5,1:100)及 7-AAD(BD Bioscience)鉴定活体 CD4 + 细胞群,FITC 通道用于检测 FoxP3 + 细胞群。每个 RNA 测序样本由 5 个同侧半球与 20 个假手术脑半球混合组成。血液样本采集于肝素抗凝管后转移至 15 毫升离心管,采用 ACK 裂解液溶解红细胞,经 70 微米滤网过滤后按上述方法进行染色。每个 RNA 测序样本由五只小鼠的样本混合制备。
Quantitative PCR 定量聚合酶链反应
RNA was extracted with RNeasy columns (QIAGEN), complementary DNA was prepared and used for qPCR (Applied Biosystems, cat. no. 437466) and the results were normalized to Gapdh (Mm99999915_g1). Applied Biosystems supplied: Il10 (cat. no. Mm01288386_m1), Il6 (cat. no. Mm00446190_m1), Tnf (cat. no. Mm00443258_m1), Il1b (cat. no. Mm00434228_m1), Il2 (cat. no. Mm00434256_m1), Il18 (cat. no. Mm00434226_m1), Ifng (cat. no. Mm01168134_m1), Bdnf (cat. no. Mm04230607_s1), Gdnf (cat. no. Mm00599849_m1), Ccl5 (cat. no. Mm01302427_m1), Cd14 (cat. no. Mm01158466_g1), Mrc1 (Cd206) (cat. no. Mn01329359_m1), Tgfb1 (cat. no. Mm01178820_m1), Il17a (cat. no. Mn00439618_m1), Cd86 (cat. no. Mm00444540_m1), Clec7a (cat. no. Mm01183349_m1), Tlr2 (cat. no. Mm00442346_m1), Cd33 (cat. no. Mm00491152_m1), Cx3cr1 (cat. no. Mm00438354_m1) and Il10ra (cat. no. Mm00434151_m1). The 2−ΔΔCt method was used to calculate the relative expression of each gene.
使用 RNeasy 柱(QIAGEN)提取 RNA,制备互补 DNA 并用于 qPCR(Applied Biosystems,货号 437466),结果以 Gapdh(Mm99999915_g1)为内参进行标准化。Applied Biosystems 提供的引物包括:Il10(货号 Mm01288386_m1)、Il6(货号 Mm00446190_m1)、Tnf(货号 Mm00443258_m1)、Il1b(货号 Mm00434228_m1)、Il2(货号 Mm00434256_m1)、Il18(货号 Mm00434226_m1)、Ifng(货号 Mm01168134_m1)、Bdnf(货号 Mm04230607_s1)、Gdnf(货号 Mm00599849_m1)、Ccl5(货号 Mm01302427_m1)、Cd14(货号 Mm01158466_g1)、Mrc1(Cd206)(货号 Mn01329359_m1)、Tgfb1(货号 Mm01178820_m1)、Il17a(货号 Mn00439618_m1)、Cd86(货号 Mm00444540_m1)、Clec7a(货号 Mm01183349_m1)、Tlr2(货号 Mm00442346_m1)、Cd33(货号 Mm00491152_m1)、Cx3cr1(货号 Mm00438354_m1)和 Il10ra(货号 Mm00434151_m1)。采用 2^-ΔΔCt 法计算各基因的相对表达量。
Isolation of primary neurons
原代神经元分离
Primary neuron isolation was carried out as previously described51. In short, primary neurons were prepared from embryos at age E18. Cell density was determined using a hemocytometer and cells were seeded. Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) was used for initial plating and the medium was changed to neurobasal supplemented with 1× B27 (Invitrogen) 3 h later. The medium was changed every 3 d.
原代神经元分离操作如前所述 51 。简言之,从胚胎第 18 天(E18)制备原代神经元。使用血细胞计数器测定细胞密度后进行接种。初始培养采用含 10%胎牛血清(FBS)的杜氏改良 Eagle 培养基(DMEM),3 小时后更换为添加 1×B27 补充剂(Invitrogen)的神经基础培养基。此后每 3 天更换一次培养基。
Induction of apoptosis and labeling of neurons
诱导细胞凋亡与神经元标记
Apoptosis and labeling of neurons were done as previously described51. Neurons were irradiated with ultraviolet light (302 nm) with an intensity of 6 × 15 W for 15 min. The apoptotic neurons were labeled with labeling dye (Alexa-405 NHS Ester, Life Technologies/Thermo Fisher Scientific). Neurons were resuspended at a density of 500,000 cells per 4 μl for stereotactic injections.
神经元凋亡及标记操作参照先前方法 51 。采用 302nm 紫外光源(强度 6×15W)照射神经元 15 分钟。凋亡神经元使用标记染料(Alexa-405 NHS 酯,Life Technologies/Thermo Fisher Scientific)进行标记。将神经元重悬至每 4μl 含 50 万个细胞的密度,用于立体定位注射。
Stereotactic injections 立体定向注射
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg kg−1). Apoptotic neurons or sterile Dulbecco’s PBS (DPBS) were injected into the lesion of TBI mice at two depths of 1.5 mm and 2 mm. Then, 2 μl was injected at each depth using stereotaxic equipment (Harvard Apparatus). After recovery from surgery, animals were returned to their cages. Post-surgery (4 or 16 h), mice were euthanized by CO2 inhalation and brains were processed for flow cytometry analysis of phagocytic microglia.
小鼠通过腹腔注射氯胺酮(100 mg/kg)麻醉。将凋亡神经元或无菌杜氏磷酸盐缓冲液(DPBS)在 1.5 毫米和 2 毫米两个深度注射入 TBI 小鼠的损伤部位。每个深度使用立体定位仪(Harvard Apparatus)注射 2 微升。术后恢复期间,动物被放回笼中。手术后(4 或 16 小时),通过二氧化碳吸入法处死小鼠,取脑组织进行流式细胞术分析小胶质细胞的吞噬功能。
Adoptive transfer 过继性转移
Splenocytes and cLNs from FoxP3-GFP or 10BiT.FoxP3GFP mice were purified and enriched using CD4 cell isolation microbead kit (Militenyi Biotech, cat. no. 130-104-454) on a magnetic MACS separator before sorting. Cell sorting was performed using FACSAriaIII cell sorter (Becton Dickson). PE anti-mouse CD3 (BioLegend, cat. no. 17A2, 1:100), APC anti-mouse CD4 (BioLegend, cat. no. GK1.5, 1:100) and 7-AAD were used to identify the live CD4+ population and the FITC channel was used to identify the FoxP3+ population. For the 10BiT.FoxP3GFP, PE anti-mouse Thy1.1 (BioLegend, cat. no. S20007C, 1:1,500) was used to identify the FoxP3+Thy1.1+ IL-10-producing FoxP3 Treg cells. All cells were injected i.p. at 2.5 million for total CD4, 1 million for CD4+FoxP3+ and 1 million for FoxP3+Thy1.1+ or FoxP3−Thy1.1− populations.
采用 CD4 细胞分离微珠试剂盒(美天旎生物技术,货号 130-104-454)在 MACS 磁珠分选仪上对 FoxP3-GFP 或 10BiT.FoxP3 小鼠的脾细胞和颈淋巴结细胞进行纯化富集后分选。使用 FACSAriaIII 流式细胞分选仪(BD 公司)进行细胞分选。PE 标记抗小鼠 CD3 抗体(BioLegend,货号 17A2,1:100)、APC 标记抗小鼠 CD4 抗体(BioLegend,货号 GK1.5,1:100)及 7-AAD 用于鉴定活体 CD4+细胞群,FITC 通道用于检测 FoxP3+细胞群。对于 10BiT.FoxP3 小鼠,采用 PE 标记抗小鼠 Thy1.1 抗体(BioLegend,货号 S20007C,1:1500)鉴定 FoxP3+Thy1.1+IL-10 分泌型 FoxP3 T 细胞。所有细胞均通过腹腔注射:总 CD4 细胞 250 万,CD4+FoxP3+细胞 100 万,FoxP3+Thy1.1+或 FoxP3+Thy1.1-细胞各 100 万。
In vitro cell culture
体外细胞培养
Sorted 4D4+ microglia 24 h post-TBI were cultured as previously described127 at 200,000 cells in a 24-well plate (Kemtec, cat. no. 4422A). The microglial culture medium was composed of 10% FBS (Gibco, cat. no. 10438026), 100 U ml−1 of penicillin–streptomycin mixture (Lonza, cat. no. DE17-602E), supplemented in DMEM/F-12 Glutamax medium (Gibco, cat. no. 10565018). FoxP3-GFP was treated with nasal aCD3 or the isotype control for 7 d and the splenic/cLN CD4+FoxP3+ and CD4+FoxP3(GFP)− population, specifically from aCD3-treated animals, was placed in a lymphocyte culture medium composed of 10% FBS, 100 U ml−1 of penicillin–streptomycin mixture, 55 µM 2-mercaptoethanol (Gibco, cat. no. 21985023), 1% sodium pyruvate (Lonza, cat. no. BE13-115E) and 1% Hepes (Lonza, cat. no. BE17-737E), supplemented in RPMI-1640 medium (Gibco, cat. no. 11875119) and then placed on the top of the hanging cell culture 0.4-µm insert (Millicell, cat. no. PTHT24H48) at 600,000 cells per insert on top of the cultured microglia, and the assay was left for 72 h in a CO2 Cell culture Incubator (InCusafe). There was a fourth group where IL-10-neutralizing antibody (BioXCell, cat. no. JES5-2A5) at a concentration of 50 μg ml−1 was added with the CD4+FoxP3+ which was cell sorted from nasal aCD3-treated mice After 72 h the microglia were lysed with buffer RLT and RNA was extracted with RNeasy columns (QIAGEN).
创伤性脑损伤后 24 小时分选的 4D4 + 小胶质细胞按前述方法 127 培养于 24 孔板(Kemtec,货号 4422A),每孔接种 200,000 个细胞。小胶质细胞培养基成分为:DMEM/F-12 Glutamax 培养基(Gibco,货号 10565018)中添加 10%胎牛血清(Gibco,货号 10438026)和 100 U/ml −1 青霉素-链霉素混合液(Lonza,货号 DE17-602E)。FoxP3-GFP 小鼠经鼻腔给予 aCD3 或同型对照处理 7 天后,取其脾脏/颈淋巴结 CD4 + FoxP3 + 与 CD4 + FoxP3(GFP) − 细胞群(仅取自 aCD3 处理组),置于淋巴细胞培养基(RPMI-1640 培养基[Gibco,货号 11875119]中添加 10%胎牛血清、100 U/ml −1 青霉素-链霉素混合液、55 µM 2-巯基乙醇[Gibco,货号 21985023]、1%丙酮酸钠[Lonza,货号 BE13-115E]和 1% Hepes[Lonza,货号 BE17-737E])中,以每孔 600,000 个细胞的密度接种于 0.4 µm 悬式细胞培养插板(Millicell,货号 PTHT24H48)中,置于已培养小胶质细胞的上层,在 CO 2 细胞培养箱(InCusafe)中共培养 72 小时。另设第四组加入 IL-10 中和抗体(BioXCell,货号 将浓度为 50 μg/ml 的 JES5-2A5 抗体与从鼻腔给予 aCD3 治疗的小鼠中分选出的 CD4+FoxP3+细胞共同培养 72 小时后,使用 RLT 裂解缓冲液裂解小胶质细胞,并通过 RNeasy 离心柱(QIAGEN)提取 RNA。
Bulk RNA-seq and analysis
批量 RNA 测序与分析
Bulk RNA-seq was performed as previously described59. Briefly, 2,000 isolated microglia CD45+CD11b+Ly6C−4D4+ were lysed in 5 μl of TCL buffer + 1% 2-mercaptoethanol. Between 800 and 1,000 isolated CD4+FoxP3(GFP+) cells from the brain and blood were processed. Smart-Seq2 libraries were prepared and sequenced using the Broad Genomic Platform. The cDNA libraries were generated from sorted cells using the Smart-seq2 protocol5. RNA-seq was performed using Illumina NextSeq500 with a High Output v.2 kit to generate reads of 2× 38 bp. Sequencing data were demultiplexed and provided by the Broad Institute in FASTQ format. The processing of the bulk RNA-seq data (transcript assembly and quantification) was based on an established computational pipeline (HISAT, Stringtie and Ballgown)128 and sequencing quality was assessed using FastQC. Reads were aligned to the ‘mm10’ reference genome. Transcript abundances were then imported into R (v.4.1.2) and converted to gene-level estimated counts using the ‘tximport’ package (v.1.22.0) from Bioconductor. Low abundance genes that achieved <10 counts aggregated across all samples were filtered out. Raw read counts were then normalized using DESeq2 (v.1.34.0). Differential gene expression was performed using DESeq2 with a significance cutoff of false discovery rate (FDR)-adjusted P < 0.05. Analyses that did not pass the FDR-correction cutoff were denoted with P < 0.05. Pairwise group comparisons were conducted using Wald’s test with standard parameters, then log2(fold-changes) were shrunk using the lfcShrink function in DESeq2. Gene expression comparisons of three or more groups were conducted using a likelihood ratio test (LRT). Heatmaps were created using ComplexHeatmap (v.2.10.1) or pheatmap (v.1.0.12) and clusters were identified via hierarchal clustering. Identified clusters were functionally annotated with Gene Ontology Biological Process (GOBP) gene-set terms using the enrichGO function of the clusterProfilerpackage (v.4.2.2), where GOBP terms with q-values < 0.05 were considered significantly enriched.
批量 RNA 测序按先前描述方法进行 59 。简言之,将 2,000 个分选的 CD45 + CD11b + Ly6C − 4D4 + 小胶质细胞裂解于 5μl 含 1% 2-巯基乙醇的 TCL 缓冲液中。对脑组织和血液中分离的 800-1,000 个 CD4 + FoxP3(GFP + )细胞进行处理。采用 Smart-Seq2 方案构建文库并在 Broad 基因组平台完成测序。分选细胞的 cDNA 文库通过 Smart-seq2 流程 5 制备。使用 Illumina NextSeq500 测序仪配合 High Output v.2 试剂盒生成 2×38 bp 读长。测序数据经 Broad 研究所解复用后以 FASTQ 格式提供。批量 RNA-seq 数据处理(转录本组装与定量)基于成熟计算流程(HISAT、Stringtie 和 Ballgown) 128 ,测序质量通过 FastQC 评估。读段比对至"mm10"参考基因组。随后将转录本丰度数据导入 R 语言(v.4.1.2),通过 Bioconductor 的"tximport"包(v.1.22.0)转换为基因水平计数。过滤掉所有样本总计数<10 的低丰度基因。 随后使用 DESeq2(v.1.34.0)对原始读数进行标准化处理。差异基因表达分析采用 DESeq2 软件,显著性阈值设定为错误发现率(FDR)校正后的 P 值<0.05。未通过 FDR 校正阈值的结果标注为 P<0.05。组间两两比较采用 Wald 检验(标准参数),随后通过 DESeq2 中的 lfcShrink 函数对 log2(倍数变化)进行收缩。三组及以上基因表达比较采用似然比检验(LRT)。热图使用 ComplexHeatmap(v.2.10.1)或 pheatmap(v.1.0.12)生成,并通过层次聚类识别簇群。鉴定出的簇群通过 clusterProfiler 包(v.4.2.2)的 enrichGO 函数进行基因本体生物过程(GOBP)功能注释,q 值<0.05 的 GOBP 条目视为显著富集。
Pathway analysis 通路分析
GSEA was performed using the clusterProfiler package and the resulting enriched terms with q-values < 0.05 were visualized through dot plots and bar plots, which were generated using the ggplot2 and ggpubr (v.0.6.0) packages. For IPA (https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa), DEGs from pairwise comparisons with corresponding log2(fold-changes) and FDR-adjusted P values were used as input. Biological networks were generated in IPA based on canonical pathways and biological functions, as outlined in our group’s previous work59.
采用 clusterProfiler 软件包进行 GSEA 分析,并通过 ggplot2 和 ggpubr(v.0.6.0)软件包生成点图和条形图来可视化 q 值<0.05 的显著富集结果。对于 IPA 分析( https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa ),输入数据为配对比较获得的差异表达基因及其对应的 log 2 (倍数变化)和 FDR 校正 P 值。如本团队先前工作所述( 59 ),基于经典通路和生物学功能在 IPA 中构建了生物网络。
Statistical and reproducibility
统计分析与可重复性
Statistical analysis was performed using GraphPad Prism v.9 software. Data are presented as mean ± s.e.m and two-sided Student’s t-tests (unpaired), two-factor, repeated-measure, two-way analysis of variance (ANOVA) (group × time) or one-way ANOVA, followed by Tukey’s multiple-comparison analysis was used to assess statistical significance between the groups. All n and P values and statistical tests are indicated in the figure legends. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications15,23. Data distribution was assumed to be normal, but this was not formally tested. Data for each experiment were collected and processed randomly and animals were assigned randomly to various experimental groups as well. The investigators were blinded to allocation during experiments and outcome assessment. No animals or data points were excluded from the analyses. Each experiment was repeated 2–3 times.
使用 GraphPad Prism v.9 软件进行统计分析。数据以均值±标准误表示,采用双侧非配对 t 检验、双因素重复测量双因素方差分析(ANOVA)(组别×时间)或单因素 ANOVA,随后通过 Tukey 多重比较分析评估组间统计学差异。所有样本量 n 值、P 值及统计检验方法均标注于图注中。未采用统计方法预先确定样本量,但样本量与既往文献报道相当 15,23 。假设数据呈正态分布但未进行正式检验。每项实验数据均采用随机方式收集处理,实验动物也随机分配至各实验组。研究人员在实验实施及结果评估阶段均采用盲法。所有实验动物及数据点均纳入分析。每项实验重复 2-3 次。
Reporting summary 报告摘要
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
关于研究设计的更多信息,请参阅本文关联的 Nature Portfolio Reporting Summary 。
Online content 在线内容
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41593-025-01877-7.
任何方法、补充参考文献、《自然》期刊报告摘要、源数据、扩展数据、补充信息、致谢内容、同行评审信息;作者贡献与利益冲突详情;以及数据和代码可用性声明均可在 10.1038/s41593-025-01877-7 获取。
Supplementary information
补充信息
Supplementary Tables 1–5.
补充表格 1-5
Acknowledgements 致谢
S.I. reports grants from the National Institute of Neurological Disorders and Stroke (grant no. 5K08NS123503-03), Department of Defense (grant no. HT9425-24-1-0635), a 2021 Neurocritical Care Society research grant and 2023 Stepping Strong Innovator Awards. S.I. and H.W. report a grant from the Department of Defense Multidisciplinary University Research Initiatives Program (grant no. W911NF-23-1-0276). We thank the NeuroTechnology Studio at Brigham and Women’s Hospital for providing instrument (Leica DMi8 Widefield Fluorescence Microscope and Zeiss LSM710 Confocal) access and consultation on data acquisition and data analysis. A Brigham Research Institute Pilot imaging microgrant was received.
S.I.报告获得以下资助:美国国家神经疾病与卒中研究所(资助编号 5K08NS123503-03)、国防部(资助编号 HT9425-24-1-0635)、2021 年神经危重症学会研究基金以及 2023 年 Stepping Strong 创新者奖。S.I.和 H.W.报告获得国防部多学科大学研究计划项目资助(资助编号 W911NF-23-1-0276)。我们感谢布莱根妇女医院神经技术工作室提供的仪器(徕卡 DMi8 宽场荧光显微镜和蔡司 LSM710 共聚焦显微镜)使用权限,以及在数据采集和分析方面的专业咨询。本研究还获得了布莱根研究所影像学试点微基金支持。
Extended data 扩展数据
Author contributions 作者贡献
S.I. designed and performed experiments and wrote the manuscript. T.Y. designed and performed experiments. R.M.R. and T.G.M. designed and supervised experiments and wrote the manuscript. M.A., H.A.E.-H., T.C., M.G.D.O., K.-J.L., M.L.B., L.C.B., D.L., W.N.B., A.C.D., T.L., F.M., J.-H.L., G.P.D.N., J.D.B., M.K. and K.C. performed experiments. R.K. sorted cells for RNA-seq experiments. O.A., P.D.S. and O.B. analyzed RNA-seq data. R.M., F.J.Q. and O.B. supervised the project. H.L.W. supervised the project and wrote the manuscript. All authors edited or commented on the manuscript. The author(s) read and approved the final manuscript.
S.I. 设计并执行实验并撰写论文。T.Y. 设计并执行实验。R.M.R. 和 T.G.M. 设计并指导实验并撰写论文。M.A.、H.A.E.-H.、T.C.、M.G.D.O.、K.-J.L.、M.L.B.、L.C.B.、D.L.、W.N.B.、A.C.D.、T.L.、F.M.、J.-H.L.、G.P.D.N.、J.D.B.、M.K. 和 K.C. 执行实验。R.K. 为 RNA 测序实验分选细胞。O.A.、P.D.S. 和 O.B. 分析 RNA 测序数据。R.M.、F.J.Q. 和 O.B. 监督项目。H.L.W. 监督项目并撰写论文。所有作者均参与论文编辑或提出修改意见。作者均已阅读并认可最终稿件。
Peer review 同行评审
Peer review information 同行评审信息
Nature Neuroscience thanks John Lukens, Edward Needham and Michal Schwartz for their contribution to the peer review of this work.
《自然-神经科学》感谢 John Lukens、Edward Needham 和 Michal Schwartz 对本论文同行评审工作作出的贡献。
Data availability 数据可用性
The Smartseq2 RNA-seq data that support the findings of the present study have been deposited into Gene Expression Omnibus under SuperSeries, accession no. GSE276761. The datasets included in the present study are available as Supplementary Data.
支持本研究结果的 Smartseq2 RNA-seq 数据已存入基因表达综合数据库(Gene Expression Omnibus)的 SuperSeries 系列,登录号为 GSE276761 。本研究所含数据集可通过 Supplementary Data 获取。
Code availability 代码可用性声明
The present study did not develop any new code. All software and packages employed are publicly accessible and fully documented in Methods.
本研究未开发新代码。所使用的所有软件及程序包均为公开资源,完整文档见 Methods 。
Competing interests 利益冲突
H.L.W. is on the scientific advisory board of Tiziana Life Sciences.
H.L.W.担任 Tiziana 生命科学公司科学顾问委员会成员。
Footnotes 脚注
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
出版者注:施普林格·自然对于出版地图和机构附属关系中的管辖权主张保持中立。
Extended data 扩展数据
is available for this paper at 10.1038/s41593-025-01877-7.
可在 10.1038/s41593-025-01877-7 获取本文补充材料
Supplementary information
补充信息
The online version contains supplementary material available at 10.1038/s41593-025-01877-7.
在线版本包含补充材料,访问地址:10.1038/s41593-025-01877-7
References 参考文献
-
1.Faul, M. & Coronado, V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol.127, 3–13 (2015).
[DOI] [PubMed] [Google Scholar]
1.Faul, M. 和 Coronado, V. 创伤性脑损伤流行病学。《临床神经病学手册》127 卷, 3-13 页 (2015 年). [ DOI ] [ PubMed ] [ Google Scholar ] -
2.Centers for Disease Control and Prevention. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014 (CDC, 2019).
2.美国疾病控制与预防中心。《创伤性脑损伤相关急诊就诊、住院及死亡监测报告——美国,2014 年》(CDC,2019 年)。 -
3.Taylor C. A., Bell, J. M., Breiding, M. J. & Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths, United States 2007 and 2013. MMWR Surveill. Summ.66, 1–16 (2017). [DOI] [PMC free article] [PubMed]
3.Taylor C. A., Bell, J. M., Breiding, M. J. 及徐立(音)。美国 2007 年与 2013 年创伤性脑损伤相关急诊就诊、住院及死亡病例分析。《发病率与死亡率周报·监测摘要》66 卷,1-16 页(2017 年)。[ DOI ][ PMC free article ][ PubMed ] -
4.Shively, S., Scher, A. I., Perl, D. P. & Diaz-Arrastia, R. Dementia resulting from traumatic brain injury: what is the pathology? Arch. Neurol.69, 1245–1251 (2012).
[DOI] [PMC free article] [PubMed] [Google Scholar]
4.Shively, S., Scher, A. I., Perl, D. P. 及 Diaz-Arrastia, R. 创伤性脑损伤所致痴呆的病理机制探讨?《神经病学档案》69 卷,1245-1251 页(2012 年)。[ DOI ][ PMC free article ][ PubMed ][ Google Scholar ] -
5.Izzy, S. et al. Association of traumatic brain injury with the risk of developing chronic cardiovascular, endocrine, neurological, and psychiatric disorders. JAMA Netw. Open5, e229478 (2022).
[DOI] [PMC free article] [PubMed] [Google Scholar]
5.Izzy, S. 等。创伤性脑损伤与慢性心血管、内分泌、神经及精神系统疾病发病风险的关联研究。《美国医学会杂志网络开放》5 卷,e229478(2022 年)。[ DOI ][ PMC free article ][ PubMed ][ Google Scholar ] -
6.Izzy, S. et al. Long-term risk of cardiovascular disease after traumatic brain injury: screening and prevention. Lancet Neurol.22, 959–970 (2023).
[DOI] [PMC free article] [PubMed] [Google Scholar]
6.Izzy, S. 等. 创伤性脑损伤后心血管疾病的长期风险:筛查与预防. 柳叶刀神经学.22, 959–970 (2023). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
7.Langlois, J. A., Rutland-Brown, W. & Wald, M. M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil.21, 375–378 (2006).
[DOI] [PubMed] [Google Scholar]
7.Langlois, J. A., Rutland-Brown, W. & Wald, M. M. 创伤性脑损伤的流行病学与影响:简要概述. 头部创伤康复杂志.21, 375–378 (2006). [ DOI ] [ PubMed ] [ Google Scholar ] -
8.Gordon, W. A. et al. Traumatic brain injury rehabilitation: state of the science. Am. J. Phys. Med. Rehabil.85, 343–382 (2006).
[DOI] [PubMed] [Google Scholar]
8.Gordon, W. A. 等. 创伤性脑损伤康复:科学现状. 美国物理医学与康复杂志.85, 343–382 (2006). [ DOI ] [ PubMed ] [ Google Scholar ] -
9.McCrory, P. et al. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. J. Athl. Train.44, 434–448 (2009).
[DOI] [PMC free article] [PubMed] [Google Scholar]
9.McCrory, P. 等. 运动相关脑震荡共识声明:2008 年 11 月苏黎世第三届国际运动脑震荡会议. 运动员训练杂志.44, 434–448 (2009). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
10.Helmick, K., Members of Consensus Conference. Cognitive rehabilitation for military personnel with mild traumatic brain injury and chronic post-concussional disorder: results of April 2009 consensus conference. NeuroRehabilitation26, 239–255 (2010).
[DOI] [PubMed] [Google Scholar]
10.Helmick, K.及共识会议成员。针对军事人员轻度创伤性脑损伤及慢性脑震荡后综合征的认知康复治疗:2009 年 4 月共识会议成果。《神经康复》26 卷,239-255 页(2010 年)。[ DOI ][ PubMed ][ Google Scholar ] -
11.Needham, E. J. et al. The immunological response to traumatic brain injury. J. Neuroimmunol.332, 112–125 (2019).
[DOI] [PubMed] [Google Scholar]
11.Needham, E. J. 等。创伤性脑损伤的免疫学应答。《神经免疫学杂志》332 卷,112-125 页(2019 年)。[ DOI ][ PubMed ][ Google Scholar ] -
12.Algattas, H. & Huang, J. H. Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int. J. Mol. Sci.15, 309–341 (2013).
[DOI] [PMC free article] [PubMed] [Google Scholar]
12.Algattas, H. 与 Huang, J. H.。创伤性脑损伤的病理生理学与治疗:损伤后早期、中期及晚期阶段。《国际分子科学杂志》15 卷,309-341 页(2013 年)。[ DOI ][ PMC free article ][ PubMed ][ Google Scholar ] -
13.Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci.8, 752–758 (2005).
[DOI] [PubMed] [Google Scholar]
13.Davalos, D. 等。ATP 介导小胶质细胞对局部脑损伤的快速体内反应。《自然神经科学》8 卷,752-758 页(2005 年)。[ DOI ][ PubMed ][ Google Scholar ] -
14.Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science308, 1314–1318 (2005).
[DOI] [PubMed] [Google Scholar]
14.Nimmerjahn, A.、Kirchhoff, F. 和 Helmchen, F. 静息态小胶质细胞在体内是高度动态的脑实质监视者。《科学》308 卷,1314–1318 页(2005 年)。[ DOI ][ PubMed ][ Google Scholar ] -
15.Roth, T. L. et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature505, 223–228 (2014).
[DOI] [PMC free article] [PubMed] [Google Scholar]
15.Roth, T. L. 等 经颅改善脑损伤后的炎症和细胞死亡。《自然》505 卷,223–228 页(2014 年)。[ DOI ][ PMC free article ][ PubMed ][ Google Scholar ] -
16.Fourgeaud, L. et al. TAM receptors regulate multiple features of microglial physiology. Nature532, 240–244 (2016).
[DOI] [PMC free article] [PubMed] [Google Scholar]
16.Fourgeaud, L. 等. TAM 受体调控小胶质细胞生理功能的多种特征. 自然 532, 240–244 (2016). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
17.Ramlackhansingh, A. F. et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol.70, 374–383 (2011).
[DOI] [PubMed] [Google Scholar]
17.Ramlackhansingh, A. F. 等. 创伤后炎症反应:小胶质细胞活化与创伤性脑损伤. 神经病学年鉴 70, 374–383 (2011). [ DOI ] [ PubMed ] [ Google Scholar ] -
18.Mannix, R. C. & Whalen, M. J. Traumatic brain injury, microglia, and Beta amyloid. Int. J. Alzheimers Dis.2012, 608732 (2012).
[DOI] [PMC free article] [PubMed] [Google Scholar]
18.Mannix, R. C. 与 Whalen, M. J. 创伤性脑损伤、小胶质细胞及β淀粉样蛋白. 国际阿尔茨海默病杂志 2012, 608732 (2012). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
19.Schimmel, S. J., Acosta, S. & Lozano, D. Neuroinflammation in traumatic brain injury: a chronic response to an acute injury. Brain Circ.3, 135–142 (2017).
[DOI] [PMC free article] [PubMed] [Google Scholar]
19.Schimmel, S. J., Acosta, S. & Lozano, D. 创伤性脑损伤中的神经炎症:急性损伤引发的慢性反应. 脑循环 3, 135–142 (2017). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
20.Jassam, Y. N., Izzy, S., Whalen, M., McGavern, D. B. & El Khoury, J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron95, 1246–1265 (2017).
[DOI] [PMC free article] [PubMed] [Google Scholar]
20.Jassam, Y. N.等. 创伤性脑损伤的神经免疫学:范式转变的时机.《神经元》95 卷, 1246–1265 页 (2017). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
21.Simon, D. W. et al. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol.13, 171–191 (2017).
[DOI] [PMC free article] [PubMed] [Google Scholar]
21.Simon, D. W.等. 创伤性脑损伤后神经炎症的深远影响.《自然综述·神经病学》13 卷, 171–191 页 (2017). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
22.Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell133, 775–787 (2008).
[DOI] [PubMed] [Google Scholar]
22.Sakaguchi, S.等. 调节性 T 细胞与免疫耐受.《细胞》133 卷, 775–787 页 (2008). [ DOI ] [ PubMed ] [ Google Scholar ] -
23.Shi, L. et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity54, 1527–1542 e1528 (2021).
[DOI] [PMC free article] [PubMed] [Google Scholar]
23.Shi, L.等. 调节性 T 细胞来源的骨桥蛋白促进小胶质细胞介导的缺血性卒中后白质修复.《免疫》54 卷, 1527–1542 页 e1528 (2021). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
24.Benakis, C. et al. T cells modulate the microglial response to brain ischemia. eLife10.7554/eLife.82031 (2022). [DOI] [PMC free article] [PubMed]
24.Benakis, C. 等. T 细胞调节小胶质细胞对脑缺血的反应. eLife10.7554/eLife.82031 (2022). [ DOI ] [ PMC free article ] [ PubMed ] -
25.Baruch, K. et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun.6, 7967 (2015).
[DOI] [PMC free article] [PubMed] [Google Scholar]
25.Baruch, K. 等. 通过靶向 Foxp3 + 调节性 T 细胞打破免疫耐受可改善阿尔茨海默病病理。Nat. Commun.6, 7967 (2015). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
26.Olson, K. E., Mosley, R. L. & Gendelman, H. E. The potential for treg-enhancing therapies in nervous system pathologies. Clin. Exp. Immunol.211, 108–121 (2023).
[DOI] [PMC free article] [PubMed] [Google Scholar]
26.Olson, K.E., Mosley, R.L. & Gendelman, H.E. 调节性 T 细胞增强疗法在神经系统疾病中的治疗潜力。临床与实验免疫学杂志 211, 108-121 (2023). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
27.Kramer, T. J. et al. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-gamma gene expression in acute experimental traumatic brain injury. J. Neuroinflam.16, 163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
27.Kramer, T. J. 等. 调节性 T 细胞的耗竭会增加急性实验性创伤性脑损伤中 T 细胞的脑部浸润、反应性星形胶质细胞增生及干扰素-γ基因表达. 神经炎症杂志 16, 163 (2019). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
28.Gao, W. et al. IL-2/anti-IL-2 complex attenuates inflammation and BBB disruption in mice subjected to traumatic brain injury. Front. Neurol.8, 281 (2017).
[DOI] [PMC free article] [PubMed] [Google Scholar]
28. 高伟等. IL-2/抗 IL-2 复合物减轻创伤性脑损伤小鼠的炎症反应及血脑屏障破坏. 神经病学前沿.8, 281 (2017). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
29.Li, M. et al. Role of regulatory T cell in clinical outcome of traumatic brain injury. Chin. Med. J.128, 1072–1078 (2015).
[DOI] [PMC free article] [PubMed] [Google Scholar]
29. 李明等. 调节性 T 细胞在创伤性脑损伤临床预后中的作用. 中华医学杂志.128, 1072–1078 (2015). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
30.Wu, H. Y., Quintana, F. J. & Weiner, H. L. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+CD25−LAP+ regulatory T cell and is associated with down-regulation of IL-17+CD4+ICOS+CXCR5+ follicular helper T cells. J. Immunol.181, 6038–6050 (2008).
[DOI] [PMC free article] [PubMed] [Google Scholar]
30. 吴海燕, Quintana, F. J. & Weiner, H. L. 鼻用抗 CD3 抗体通过诱导分泌 IL-10 的 CD4 + CD25 − LAP + 调节性 T 细胞改善狼疮症状,并与 IL-17 + CD4 + ICOS + CXCR5 + 滤泡辅助性 T 细胞的下调相关. 免疫学杂志.181, 6038–6050 (2008). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
31.Mayo, L. et al. IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation. Brain139, 1939–1957 (2016).
[DOI] [PMC free article] [PubMed] [Google Scholar]
31. 梅奥·L 等. IL-10 依赖性 Tr1 细胞可减轻星形胶质细胞活化并改善慢性中枢神经系统炎症. 脑.139, 1939–1957 (2016). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
32.Lopes, J. R. et al. Nasal administration of anti-CD3 monoclonal antibody ameliorates disease in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA120, e2309221120 (2023).
[DOI] [PMC free article] [PubMed] [Google Scholar]
32.Lopes, J. R. 等. 鼻腔给予抗 CD3 单克隆抗体可改善阿尔茨海默病小鼠模型的疾病症状. 美国国家科学院院刊 120, e2309221120 (2023). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] - 33.Smith, D. H. et al. Pre-clinical traumatic brain injury common data elements: toward a common language across laboratories. J. Neurotrauma32, 1725–1735 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner, A. K. et al. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res.998, 113–121 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Monaco, C. M. et al. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol.247, 410–418 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong, Y. et al. Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res.1185, 301–312 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam, A. et al. Cellular infiltration in traumatic brain injury. J. Neuroinflam.17, 328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter, S. et al. Serum biomarkers identify critically ill traumatic brain injury patients for MRI. Criti. Care26, 369 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van, V. Q. et al. Cutting edge: CD47 controls the in vivo proliferation and homeostasis of peripheral CD4+ CD25+ Foxp3+ regulatory T cells that express CD103. J. Immunol.181, 5204–5208 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Layman, A. A. K. et al. Ndfip1 restricts mTORC1 signalling and glycolysis in regulatory T cells to prevent autoinflammatory disease. Nat. Commun.8, 15677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashiwakura, Y. et al. CD2-mediated regulation of peripheral CD4+CD25+ regulatory T-cell apoptosis accompanied by down-regulation of Bim. Immunology139, 48–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing, S. et al. Tcf1 and Lef1 are required for the immunosuppressive function of regulatory T cells. J. Exp. Med.216, 847–866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garin, M. I. et al. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood109, 2058–2065 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Ono, M. et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature446, 685–689 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Ohkura, N. & Sakaguchi, S. Foxo1 and Foxo3 help Foxp3. Immunity33, 835–837 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Fu, W. et al. Foxo4- and Stat3-dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis. FASEB J.31, 1354–1367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain, N. et al. Cutting edge: Dab2 is a FOXP3 target gene required for regulatory T cell function. J. Immunol.183, 4192–4196 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He, F. et al. PLAU inferred from a correlation network is critical for suppressor function of regulatory T cells. Mol. Syst. Biol.8, 624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Probst-Kepper, M. et al. GARP: a key receptor controlling FOXP3 in human regulatory T cells. J. Cell. Mol. Med.13, 3343–3357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmetterer, K. G. & Pickl, W. F. The IL-10/STAT3 axis: contributions to immune tolerance by thymus and peripherally derived regulatory T-cells. Eur. J. Immunol.47, 1256–1265 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Krasemann, S. et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity47, 566–581 e569 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickman, S., Izzy, S., Sen, P., Morsett, L. & El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci.21, 1359–1369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci.16, 1896–1905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crocker, P. R., McMillan, S. J. & Richards, H. E. CD33-related siglecs as potential modulators of inflammatory responses. Ann. N. Y. Acad. Sci.1253, 102–111 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Crocker, P. R., Paulson, J. C. & Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol.7, 255–266 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Workman, C. J. & Vignali, D. A. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur. J. Immunol.33, 970–979 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Janova, H. et al. CD14 is a key organizer of microglial responses to CNS infection and injury. Glia64, 635–649 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Zoller, T. et al. Silencing of TGFbeta signalling in microglia results in impaired homeostasis. Nat. Commun.9, 4011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butovsky, O. et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci.17, 131–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor, R. A. et al. TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Invest.127, 280–292 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keren-Shaul, H. et al. A unique nicroglia type associated with restricting development of Alzheimer’s disease. Cell169, 1276–1290.e1217 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Miranda, M., Morici, J. F., Zanoni, M. B. & Bekinschtein, P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front. Cell Neurosci.13, 363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neher, J. J. et al. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc. Natl Acad. Sci. USA110, E4098–E4107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Healy, L. M. et al. MerTK Is a functional regulator of myelin phagocytosis by human myeloid cells. J. Immunol.196, 3375–3384 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Wu, C., Yang, L., Youngblood, H., Liu, T. C. & Duan, R. Microglial SIRPalpha deletion facilitates synapse loss in preclinical models of neurodegeneration. Neurosci. Bull.38, 232–234 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajbhandari, L. et al. Toll-like receptor 4 deficiency impairs microglial phagocytosis of degenerating axons. Glia62, 1982–1991 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Park, S. Y. & Kim, I. S. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp. Mol. Med.49, e331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li, W. Eat-me signals: keys to molecular phagocyte biology and ‘appetite’ control. J. Cell. Physiol.227, 1291–1297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moon, B., Yang, S., Moon, H., Lee, J. & Park, D. After cell death: the molecular machinery of efferocytosis. Exp. Mol. Med.55, 1644–1651 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dou, Y. et al. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res.22, 1022–1033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amick, J., Roczniak-Ferguson, A. & Ferguson, S. M. C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol. Biol. Cell27, 3040–3051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smits, D. J. et al. CLEC16A interacts with retromer and TRIM27, and its loss impairs endosomal trafficking and neurodevelopment. Hum. Genet.142, 379–397 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jonker, C. T. H. et al. Vps3 and Vps8 control integrin trafficking from early to recycling endosomes and regulate integrin-dependent functions. Nat. Commun.9, 792 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang, N. et al. Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination. Mol. Neurodegener.17, 75 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filipello, F. et al. Defects in lysosomal function and lipid metabolism in human microglia harboring a TREM2 loss of function mutation. Acta Neuropathol.145, 749–772 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi, Y. et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron109, 2413–2426.e2417 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barger, S. R. et al. Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat. Commun.10, 1249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fairley, L. H. et al. Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease. Proc. Natl Acad. Sci. USA120, e2209177120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren, J. et al. Qki is an essential regulator of microglial phagocytosis in demyelination. J. Exp. Med.10.1084/jem.20190348 (2021). [DOI] [PMC free article] [PubMed]
- 80.Schmidt, C. et al. Phosphoinositide 3-kinase gamma mediates microglial phagocytosis via lipid kinase-independent control of cAMP. Neuroscience233, 44–53 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Karaca, N. E. et al. Early diagnosis and hematopoietic stem cell transplantation for IL10R deficiency leading to very early-onset inflammatory bowel disease are essential in familial cases. Case Rep. Immunol.2016, 5459029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laffer, B. et al. Loss of IL-10 promotes differentiation of microglia to a M1 phenotype. Front. Cell Neurosci.13, 430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loftspring, M. C., McDole, J., Lu, A., Clark, J. F. & Johnson, A. J. Intracerebral hemorrhage leads to infiltration of several leukocyte populations with concomitant pathophysiological changes. J. Cereb. Blood Flow Metab.29, 137–143 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mracsko, E. et al. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke45, 2107–2114 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Guo, F. Q. et al. Study of relationship between inflammatory response and apoptosis in perihematoma region in patients with intracerebral hemorrhage]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue18, 290–293 (2006). [PubMed] [Google Scholar]
- 86.Zhang, W., Wu, Q., Hao, S. & Chen, S. The hallmark and crosstalk of immune cells after intracerebral hemorrhage: Immunotherapy perspectives. Front. Neurosci.16, 1117999 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yshii, L. et al. Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat. Immunol.23, 878–891 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maynard, C. L. et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat. Immunol.8, 931–941 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Izzy, S. et al. Time-dependent changes in microglia transcriptional networks following traumatic brain injury. Front. Cell Neurosci.13, 307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Houlton, J., Abumaria, N., Hinkley, S. F. R. & Clarkson, A. N. Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front. Neurosci.13, 790 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu, L. et al. T-cell infiltration, contribution and regulation in the central nervous system post-traumatic injury. Cell Prolif.54, e13092 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Curotto de Lafaille, M. A. & Lafaille, J. J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity30, 626–635 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Garcia, J. M. et al. Role of interleukin-10 in acute brain injuries. Front. Neurol.8, 244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiemessen, M. M. et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl Acad. Sci. USA104, 19446–19451 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao, W., Beers, D. R., Liao, B., Henkel, J. S. & Appel, S. H. Regulatory T lymphocytes from ALS mice suppress microglia and effector T lymphocytes through different cytokine-mediated mechanisms. Neurobiol. Dis.48, 418–428 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strle, K. et al. Interleukin-10 in the brain. Crit. Rev. Immunol.21, 427–449 (2001). [PubMed] [Google Scholar]
- 97.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol.19, 683–765 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Schneider Soares, F. M. et al. Interleukin-10 is an independent biomarker of severe traumatic brain injury prognosis. Neuroimmunomodulation19, 377–385 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Kirchhoff, C. et al. Cerebrospinal IL-10 concentration is elevated in non-survivors as compared to survivors after severe traumatic brain injury. Eur. J. Med. Res.13, 464–468 (2008). [PubMed] [Google Scholar]
- 100.Chen, X. et al. Interleukin-10 mediates the neuroprotection of hyperbaric oxygen therapy against traumatic brain injury in mice. Neuroscience266, 235–243 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Kline, A. E. et al. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res.937, 22–31 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Lee, H. F., Lee, T. S. & Kou, Y. R. Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respir. Physiol. Neurobiol.182, 1–8 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Caplan, H. W. et al. Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy. Stem Cells39, 358–370 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chitnis, T. et al. Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects. Front. Immunol.13, 956907 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moreira, T. G. et al. Nasal administration of anti-CD3 monoclonal antibody (foralumab) reduces lung inflammation and blood inflammatory biomarkers in mild to moderate COVID-19 patients: a pilot study. Front. Immunol.12, 709861 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moreira, T. G. et al. Nasal administration of anti-CD3 mAb (foralumab) downregulates NKG7 and increases TGFB1 and GIMAP7 expression in T cells in subjects with COVID-19. Proc. Natl Acad. Sci. USA120, e2220272120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hickman, S. E. & El Khoury, J. Analysis of the microglial sensome. Methods Mol. Biol.2034, 305–323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galatro, T. F. et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci.20, 1162–1171 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity40, 274–288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaiser, T. & Feng, G. Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro10.1523/ENEURO.0448-18.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bermpohl, D., You, Z., Lo, E. H., Kim, H. H. & Whalen, M. J. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab.27, 1806–1818 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Kraeuter, A. K., Guest, P. C. & Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol. Biol.1916, 99–103 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc.1, 848–858 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu, L. et al. Repetitive head injury in adolescent mice: a role for vascular inflammation. J. Cereb. Blood Flow Metab.39, 2196–2209 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jarvis, B. B. et al. New macrocyclic trichothecenes from Baccharis megapotamica. J. Nat. Prod.50, 815–828 (1987). [DOI] [PubMed] [Google Scholar]
- 116.Natarajan, R., Northrop, N. & Yamamoto, B. Fluorescein isothiocyanate (FITC)-Dextran extravasation as a measure of blood-brain barrier permeability. Curr. Protoc. Neurosci.79, 9.58.1–59.58.15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature509, 507–511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li, J. et al. Retro-orbital injection of FITC-dextran combined with isolectin B4 in assessing the retinal neovascularization defect. BMC Ophthalmol.21, 208 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li, S. et al. Retro-orbital injection of FITC-dextran is an effective and economical method for observing mouse retinal vessels. Mol. Vis.17, 3566–3573 (2011). [PMC free article] [PubMed] [Google Scholar]
- 120.Yang, S. et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front. Immunol.8, 902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu, L. et al. Genetic inhibition of RIPK3 ameliorates functional outcome in controlled cortical impact independent of necroptosis. Cell Death Dis.12, 1064 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fedorov, A. et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging30, 1323–1341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Louveau, A., Filiano, A. J. & Kipnis, J. Meningeal whole mount preparation and characterization of neural cells by flow cytometry. Curr. Protoc. Immunol.121, e50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Izzy, S. et al. Repetitive traumatic brain injury causes neuroinflammation before tau pathology in adolescent P301S mice. Int. J. Mol. Sci.22, 907 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mallory, K. L. et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines6, 84 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rezende, R. M. et al. gammadelta T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun.9, 3151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xie, L., Choudhury, G. R., Winters, A., Yang, S. H. & Jin, K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur. J. Immunol.45, 180–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pertea, M., Kim, D., Pertea, G. M., Leek, J. T. & Salzberg, S. L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc.11, 1650–1667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–5.
Data Availability Statement
The Smartseq2 RNA-seq data that support the findings of the present study have been deposited into Gene Expression Omnibus under SuperSeries, accession no. GSE276761. The datasets included in the present study are available as Supplementary Data.
The present study did not develop any new code. All software and packages employed are publicly accessible and fully documented in Methods.