Abstract 抽象
Organoids are simple tissue-engineered cell-based in vitro models that recapitulate many aspects of the complex structure and function of the corresponding in vivo tissue. They can be dissected and interrogated for fundamental mechanistic studies on development, regeneration and repair in human tissues, and can also be used in diagnostics, disease modelling, drug discovery and personalized medicine. Organoids are derived from either pluripotent or tissue-resident stem (embryonic or adult) or progenitor or differentiated cells from healthy or diseased tissues, such as tumours. To date, numerous organoid engineering strategies that support organoid culture and growth, proliferation, differentiation and maturation have been reported. This Primer highlights the rationale underlying the selection and development of these materials and methods to control the cellular/tissue niche; and therefore, the structure and function of the engineered organoid. We also discuss key considerations for generating robust organoids, such as those related to cell isolation and seeding, matrix and soluble factor selection, physical cues and integration. The general standards for data quality, reproducibility and deposition within the organoid community are also outlined. Lastly, we conclude by elaborating on the limitations of organoids in different applications, and the key priorities in organoid engineering for the coming years.
类器官是简单的组织工程细胞体外模型,概括了相应体内组织的复杂结构和功能的许多方面。它们可以被解剖和询问,用于人体组织发育、再生和修复的基本机制研究,也可用于诊断、疾病建模、药物发现和个性化医疗。类器官来源于多能或组织驻留的干(胚胎或成体)或祖细胞或来自健康或患病组织(如肿瘤)的分化细胞。迄今为止,已经报道了许多支持类器官培养和生长、增殖、分化和成熟的类器官工程策略。本入门强调了选择和开发这些材料和方法以控制细胞/组织生态位的基本原理;因此,工程类器官的结构和功能。我们还讨论了生成稳健类器官的关键考虑因素,例如与细胞分离和接种、基质和可溶性因子选择、物理线索和整合相关的考虑因素。还概述了类器官群落内数据质量、可重复性和沉积的一般标准。最后,我们详细阐述了类器官在不同应用中的局限性,以及未来几年类器官工程的关键优先事项。
Similar content being viewed by others
其他人正在查看类似内容
Introduction 介绍
Stem cells are critical in maintaining organ size, structure and function through cellular renewal, migration, differentiation and apoptosis1. Stem cells reside in a defined microenvironment commonly referred to as the stem cell niche to regulate stem cell fate2. Given the importance of these environmental cues, there have been numerous tissue engineering attempts to mimic the stem cell niche in vitro to achieve high spatio-temporal control over cell–cell and cell–matrix interactions and reproduce mechano-chemical cues using engineered hydrogels and micro-devices3,4. In 1977, Matrigel, a basement membrane extracellular matrix (ECM) containing a unique mix of ECM components and growth factors, was extracted from mouse sarcoma tumours and used to support in vitro cell culture5. Matrigel was later shown to allow breast epithelial cells to grow in three dimensions and form lumens with milk protein secretion6, and adult intestinal stem cells embedded in Matrigel in the presence of a tissue-specific cocktail of growth factors were also shown to self-organize into 3D crypt–villus structures7. Organoid research intertwined with 3D cell culture, stem cell and tissue engineering for over a century, with various debates on the definition, standard and scope.
干细胞通过细胞更新、迁移、分化和细胞凋亡来维持器官大小、结构和功能至关重要 1。干细胞驻留在一个定义的微环境中,通常称为 干细胞生态位 ,以调节干细胞命运 2。鉴于这些环境线索的重要性,已经有许多组织工程尝试在体外模拟干细胞生态位,以实现对细胞-细胞和细胞-基质相互作用的高度时空控制,并使用工程水凝胶和微型设备再现机械化学线索 3,4。1977 年,从小鼠肉瘤肿瘤中提取了基底膜细胞外基质 (ECM),其中含有 ECM 成分和生长因子的独特混合物,用于支持体外细胞培养 5。基质胶后来被证明允许乳腺上皮细胞在三维空间中生长,并与牛奶蛋白分泌形成管腔 6,在存在组织特异性生长因子混合物的情况下,嵌入基质胶中的成体肠道干细胞也被证明可以自组织成 3D 隐窝-绒毛结构 7.一个多世纪以来,类器官研究与 3D 细胞培养、干细胞和组织工程交织在一起,在定义、标准和范围方面存在各种争论。
An organoid is a self-organized 3D tissue that is typically derived from stem cells (pluripotent, fetal or adult), and which mimics the key functional, structural and biological complexity of an organ8,9,10,11. Cells comprising organoids can be derived from induced pluripotent stem cells (iPSCs) or tissue-derived cells (TDCs), including normal stem/progenitor cells, differentiated cells and cancer cells12. Compared with conventional 2D cultures and animal models, organoid cultures enable patient specificity in the model while recapitulating in vivo tissue-like structures and functions in vitro. Organoid cultures are more accessible for manipulation and in-depth biological studies13 than animal models. As such, organoid cultures have been leveraged for a wide variety of applications including drug discovery14,15, personalized companion diagnostics15 and cell therapy13.
类器官是一种自组织的 3D 组织,通常来源于干细胞(多能细胞,胎儿或成人),它模仿器官的关键功能,结构和生物学复杂性 8,9,10,11。包含类器官的细胞可以来源于诱导多能干细胞(iPSC)或组织来源细胞(TDC),包括正常干细胞/祖细胞,分化细胞和癌细胞 12。与传统的 2D 培养物和动物模型相比,类器官培养物在模型中具有患者特异性,同时在体外概括体内组织样结构和功能。与动物模型相比,类器官培养物更容易用于作和深入的生物学研究 13。因此,类器官培养已被用于广泛的应用,包括药物发现 14,15,个性化伴随诊断 15 和细胞治疗 13。
Organoid cultures exhibit significant heterogeneity and variable complexity in cellular composition, can undergo poorly controlled morphogenesis in the self-assembly process and often lack stromal, vascular and immunological components4,12. Hence, there is a great need to improve organoid culture by leveraging our understanding of organogenesis as well as how cells interact with their cellular and physical microenvironment in the form of the stem cell niche. Based on these insights, bioengineering strategies could be developed to precisely control stem cell decisions during organoid development. For example, from early embryogenesis studies, it is known that morphogen gradients regulate tissue patterning and development16,17. Microfluidics systems can be used to create the required concentration gradients of these by diffusing morphogens, giving rise to the desired cell types with spatial patterning16. Beyond biochemical cues, stem cells also experience active and passive forces from their external microenvironment and convert these physical stimuli into biochemical responses18. These physical cues arise from the matrix, external forces and/or cell–cell interactions. Rather than relying on a natural or biologically derived ECM such as Matrigel with limited stiffness tunability, synthetic hydrogels or other ECM combinations can be leveraged to control the physical properties of the matrix. Liquid friction against the cell membrane can also exert shear stress on cells19. The dynamic biofluidic environment has diverse effects on different cell types depending on the magnitude, direction and frequency19. Hence, microfluidic systems and bioreactors can be applied to provide perfusion at both the micro-scale and macro-scale20,21,22. Lastly, it is now known that cells interact with their neighbours and respond to external stimuli in a collective manner23; topographical cues, such as the curvature and shape of neighbouring cells, can affect stem cell decisions24. A recent neural tube model dissected the folding process and demonstrated that geometry constraints by micropatterning can control the final morphology of neural tube-like structures25.
类器官培养物在细胞组成上表现出显着的异质性和可变的复杂性,在自组装过程中可能经历控制不佳的形态发生,并且通常缺乏基质、血管和免疫学成分 4,12。因此,非常需要利用我们对 器官发生的理解以及细胞如何以干细胞生态位的形式与其细胞和物理微环境相互作用来改进类器官培养。基于这些见解,可以开发生物工程策略来精确控制类器官发育过程中的干细胞决策。例如,从早期胚胎发生研究中,已知形态原梯度调节组织模式和发育 16,17。微流体系统可用于通过扩散形态原来产生所需的浓度梯度,从而产生具有空间图案 16 的所需细胞类型。除了生化线索之外,干细胞还经历来自外部微环境的主动和被动力量,并将这些物理刺激转化为生化反应 18。这些物理线索源于基质、外力和/或细胞间相互作用。与其依赖天然或生物衍生的 ECM(例如刚度可调性有限的基质胶),不如利用合成水凝胶或其他 ECM 组合来控制基质的物理性能。与细胞膜的液体摩擦也会对细胞施加剪切应力 19。动态生物流控环境根据大小、方向和频率对不同细胞类型有不同的影响 19。 因此,微流体系统和生物反应器可用于提供微观和宏观尺度的灌注 20,21,22。最后,现在已知细胞与其邻居相互作用并以集体方式对外部刺激做出反应 23;地形线索,例如相邻细胞的曲率和形状,会影响干细胞的决策 24。最近的神经管模型剖析了折叠过程,并证明微图案化的几何约束可以控制神经管状结构的最终形态 25。
It is debatable whether engineered cell-based in vitro models such as organoids need to faithfully recapitulate the structures and functions of the in vivo organ of origin. One trend is to recapitulate as much in vivo tissue architecture and function as possible in vitro in order to demonstrate the physiological relevance of models of increasing complexity. For bioengineers, the artificially created in vitro models only need to recapitulate specific features of the in vivo tissue, relevant to the physiological or diseased functions of interest. There is an optimism to creating highly complex models and expecting them to accurately mimic the in vivo organ of origin. For the majority of users, simpler models — such as a model with one or two cells in monolayer or 3D culture — are more robust for mechanistic studies and applications26,27,28 than more complex models, such as assembloids, or other multicellular models.
基于工程细胞的体外模型(例如类器官)是否需要忠实地概括体内起源器官的结构和功能,这是值得商榷的。一种趋势是在体外尽可能多地概括体内组织结构和功能,以证明日益复杂的模型的生理相关性。对于生物工程师来说,人工创建的体外模型只需要概括体内组织的特定特征,与感兴趣的生理或疾病功能相关。人们对创建高度复杂的模型并期望它们准确模仿体内起源器官持乐观态度。对于大多数用户来说,更简单的模型(例如在单层或 3D 培养中具有一个或两个细胞的模型)比更复杂的模型(例如 组合体或其他多细胞模型)更稳健 26,27,28。
In this Primer, we focus on the rationale underlying the establishment of organoid cultures and provide guiding principles for the selection of suitable materials and methods for different applications. We first discuss the experimental considerations for setting up organoid-based cultures, categorized into four major components — cells, soluble factors, matrix and physical cues — and discuss approaches to integrate these components (Fig. 1). We also discuss key considerations for generating more complex yet robust organoids, such as those related to cell isolation and seeding, matrix and soluble factor selection, physical cues and integration. The general standards for data quality, reproducibility and deposition within the organoid community are also outlined. Lastly, we conclude by elaborating on the limitations of organoids in different applications, and the key priorities in organoid engineering for the coming years.
在本入门中,我们重点介绍了建立类器官培养物的基本原理,并为为不同应用选择合适的材料和方法提供了指导原则。我们首先讨论建立基于类器官的培养物的实验注意事项,分为四个主要组成部分——细胞、可溶性因子、基质和物理线索——并讨论整合这些成分的方法(图。1). 我们还讨论了生成更复杂但稳健的类器官的关键考虑因素,例如与细胞分离和接种、基质和可溶性因子选择、物理线索和整合相关的考虑因素。还概述了类器官群落内数据质量、可重复性和沉积的一般标准。最后,我们详细阐述了类器官在不同应用中的局限性,以及未来几年类器官工程的关键优先事项。
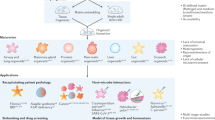
图1:类器官工程的组成部分。
The establishment of organoid-based culture requires considerations about major components that make up organoid cultures — cells, soluble factors and matrix, physical cues — and the successful integration of these components. ASCs, adult stem cells; CSCs, cancer stem cells; ECM, extracellular matrix; FGF, fibroblast growth factor; iPSCs, induced pluripotent stem cells; OoC, organ-on-a-chip; TDCs, tissue-derived cells.
基于类器官的培养物的建立需要考虑构成类器官培养物的主要成分——细胞、可溶性因子和基质、物理线索——以及这些成分的成功整合。ASC,成体干细胞;CSC,癌症干细胞;ECM,细胞外基质;FGF,成纤维细胞生长因子;iPSC,诱导多能干细胞;OoC,器官芯片;TDC,组织来源的细胞。
Experimentation 实验
Cell source 细胞来源
Under defined physicochemical conditions, tissues such as small intestine7, colon29,30, stomach31,32, oesophagus29, tongue33, liver34,35,36,37, lung14, pancreas38,39,40, heart41, ear42 and skin43 have been obtained from iPSCs, adult or fetal cells and either stem/progenitor cells or differentiated cells. The starting cellular population for any given organoid is of prime importance, affecting not only the variability and heterogeneity in the structures obtained but also the function of the tissue they aim to model. To establish tissue-derived organoids or cancer organoids, tissue-resident stem/progenitor/differentiated cells or tumour cells, respectively, are obtained through an optimized tissue dissociation method. For iPSC-derived organoids, iPSC lines are established and fully characterized as the starting cells. Patient/tissue-derived stem cells are obtained through an optimized tissue dissociation method and then embedded into a 3D matrix mimicking stem cell niches. iPSCs can be maintained and expanded as undifferentiated clonal populations on feeder cells. To exemplify the generation of tissue-derived organoids we use intestinal organoids as an example (Fig. 2a), as this was the first tissue-derived organoid type established7. The small intestine and colon are opened longitudinally, washed and then cut into 2–4 mm fragments to increase the surface area for enzymatic digestion or further mechanical dissociation. EDTA treatment is used to chelate calcium, disrupting cell–cell adhesion and tissue integrity44. Larger tissue fragments and whole cells are removed from collected crypt fractions, and the harvested primary intestinal crypts are used for seeding and generation of intestinal organoid cultures.
在规定的理化条件下,小肠 7、结肠 29、30、胃 31、32、食道 29、舌头 33、肝脏 34、35、36、37、肺 14、胰腺 38、39、40、心脏 41、耳朵 42 和皮肤 43 已从 iPSC、成人或胎儿细胞以及干细胞/祖细胞或分化细胞中获得。任何给定类器官的起始细胞群都至关重要,不仅影响所获得结构的变异性和异质性,还影响它们旨在建模的组织的功能。为了建立组织来源的类器官或癌症类器官,分别通过优化的组织解离方法获得组织驻留干细胞/祖细胞/分化细胞或肿瘤细胞。对于 iPSC 衍生的类器官,iPSC 系被建立并完全表征为起始细胞。患者/组织来源的干细胞通过优化的组织解离方法获得,然后嵌入到模仿干细胞生态位的 3D 矩阵中。iPSC 可以作为饲养细胞上的未分化克隆群体进行维持和扩增。为了举例说明
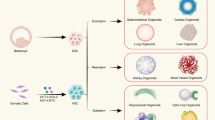
图2:程序流程图。
Organoids can be generated from tissue-derived cells (TDCs) or induced pluripotent stem cells (iPSCs). a, To generate organoids from TDCs, tissue samples are obtained from humans or animals, such as the gut and stomach. The intestinal tissue samples are opened, washed and then cut into small fragments (2–4 mm) to increase the surface area for enzymatic digestion or further mechanical dissociation to isolate single intestinal stem cells or crypts. After several rounds of washing and purification, the harvested stem cells or crypts will be used for seeding and generation of organoid cultures for expansion. b, To generate organoids from iPSCs using genetic engineering, iPSCs are maintained and expanded as undifferentiated clonal populations on feeder cells or defined extracellular matrix (ECM) substrates to aggregate to embryoid bodies. Typically, iPSCs are harvested as cell aggregates, which preserve cell–cell contact and yield cell populations with higher viability. These aggregates are further induced through germ layer specification to form endodermal spheres, mesodermal domes and neuroectodermal matrix for additional applications.
类器官可以由组织衍生细胞 (TDC) 或诱导多能干细胞 (iPSC) 产生。a,为了从 TDC 生成类器官,从人类或动物(例如肠道和胃)中获取组织样本。打开、洗涤肠组织样品,然后切成小碎片(2-4 mm),以增加酶消化或进一步机械解离的表面积,以分离单个肠干细胞或隐窝。经过几轮洗涤和纯化后,收获的干细胞或隐窝将用于接种和产生类器官培养物以进行扩增。b,为了使用基因工程从 iPSCs 中产生类器官,iPSC 被维持和扩增为饲养细胞或定义的细胞外基质(ECM)底物上的未分化克隆群,以聚集到胚样体中。通常,iPSC 作为细胞聚集体收获,以保持细胞间接触并产生具有更高活力的细胞群。这些聚集体通过胚层规格进一步诱导,形成内胚层球体、中胚层圆顶和神经外胚层基质,以用于其他应用。
The starting cellular populations for organoid cultures are generally obtained from adult or fetal tissue biopsy samples. The most commonly used tissue dissociation method is enzymatic digestion, which dissolves the ECM45. The composition of the enzymatic cocktail and the efficacy of the enzymatic dissociation process varies with tissue type46, and in certain cases DNase can be added to remove excessive DNA released from necrotic cells47. Depending on the tissue type, the tissue fragments can be further incubated with enzymes such as collagenase, elastase or dispase to generate single-cell suspensions and then seeded in Matrigel. The enzymatic dissociation method may affect the cell state of retrieved cells as it may require extended durations in the enzymatic mix to dissociate the majority of the tissue-resident stem cells. Tissue dissociation can also be achieved mechanically; although mechanical dissociation is much faster and less expensive, the cell yield and viability can be inconsistent46. Mechanical and enzymatic dissociation can be combined to generate better cell yield. After tissue dissociation, TDCs for organoid development are identified and collected using known biomarkers or physical characteristics45. Tissue-specific stem cell markers are typically used to identify and isolate the desired stem cells to generate organoids1,2. Fluorescence-activated cell sorting or magnetic-activated cell sorting isolates cells based on multiple parameters, including size, shape and cell-surface marker expression45,46. Other isolation techniques include laser capture microdissection and manual cell picking45.
iPSCs can be maintained and expanded as undifferentiated clonal populations over many generations. Undifferentiated human iPSCs are typically maintained on feeder cells or defined ECM substrates. As single iPSCs do not survive well in vitro, iPSCs are typically harvested as cell aggregates, which preserve cell–cell contact, yielding cell populations with higher viability. Physical scraping can also compensate for the lack of uniformity of cell aggregates. The dissociation enzymatic mixture should be chosen based on the level of cell sensitivity44 and whether the cultured cells secrete excessive ECM, making it difficult to detach the cells from the cell culture plate (Fig. 2b).
Tumour tissue, derived from either biopsy samples or surgical resections, is also typically processed akin to normal tissue to isolate tumour cells to grow as organoids15,48,49,50. Tumour cells isolated from liquid samples such as peripheral blood51, ascites49,52 and pleural effusions53 can be used as starting material to generate organoids. Patient-derived tumour organoids can be generated from samples obtained from minimally invasive Pap brush material54,55. Owing to their low numbers, tumour cells from biopsy samples or liquid samples can be first expanded in animal models as xenografts in order to obtain sufficient cells for organoid generation56. In the case of tumour tissue, it is preferable to limit tissue dissociation so that cell clusters rather than single cells are isolated. A critical factor that can influence the generation of tumour-derived organoids is the fact that isolated cells from tissue typically contain both cancer and normal cells. Although for some tumours it may be possible to enrich for tumour-forming cells by sorting a priori57, for the majority there is currently no robust method to separate normal and tumour cell populations prior to seeding into a matrix for culture. An approach to overcome this issue is to take advantage of culture conditions by using selective media that omits certain factors required for growth of normal organoids, as tumour cells gradually lose dependence on those factors during malignant transformation58. Blood contamination, particularly erythrocytes, can also affect organoid generation and matrix stability, and therefore standard approaches to eliminate these through lysis are typically used59.
来自活检样本或手术切除的肿瘤组织通常也像正常组织一样进行处理,以分离肿瘤细胞以生长为类器官 15,48,49,50。从液体样本(如外周血 51,腹水 49,52 和胸腔积液 53)中分离的肿瘤细胞可用作产生类器官的起始材料。患者来源的肿瘤类器官可以从从微创巴氏刷材料 54,55 获得的样品中产生。由于数量少,活检样本或液体样本中的肿瘤细胞可以首先在动物模型中扩增为异种移植物,以获得足够的细胞用于类器官的生成 56。在肿瘤组织的情况下,最好限制组织解离,以便分离细胞簇而不是单个细胞。影响肿瘤来源类器官生成的一个关键因素是,从组织中分离的细胞通常同时含有癌症细胞和正常细胞。尽管对于某些肿瘤,可以通过先验分选 57 来富集肿瘤形成细胞,但对于大多数肿瘤来说,目前没有可靠的方法在接种到基质中进行培养之前分离正常细胞群和肿瘤细胞群。克服这个问题的一种方法是通过使用省略正常类器官生长所需的某些因素的选择性培养基来利用培养条件,因为肿瘤细胞在恶性转化过程中逐渐失去对这些因素的依赖性 58。 血液污染,特别是红细胞,也会影响类器官的生成和基质稳定性,因此通常使用通过裂解消除这些污染的标准方法 59。
Matrix 矩阵
Following cell isolation, cells are typically seeded into biologically derived matrices such as Matrigel7,60 or a natural ECM such as collagen61, or into synthetic hydrogels3,62,63. Matrigel is mainly composed of laminin, collagen IV, entactin, perlecan and growth factors, and is similar in composition to the basement membrane61. As a continuation from the above example using intestinal organoids, we now briefly describe how cells are encapsulated into matrices. Isolated intestinal crypts are first re-suspended into cold Matrigel and pipetted into pre-warmed low-attachment well plates for culture, producing a flat semi-sphere gel of cell–matrix construct. The complete composition of intestinal organoid culture medium has been previously reported29. Organoids go through passaging, and the proliferation rate is measured by isolating organoids from the Matrigel, removing single cells and counting crypts. The expansion rate is calculated as the number of organoids from each well divided by the number of initial crypts seeded in Matrigel in that well.
细胞分离后,通常将细胞接种到生物来源的基质(如基质胶 7,60)或天然 ECM(如胶原蛋白 61)中,或接种到合成水凝胶 3,62,63 中。基质胶主要由层粘连蛋白、IV 型胶原蛋白、内肌蛋白、基底膜聚糖和生长因子组成,其成分与基底膜 61 相似。作为上述使用肠道类器官的示例的延续,我们现在简要描述
Although Matrigel can support organoid culture, the inherently heterogeneous and poorly defined composition of this biologically derived matrix offers little control over the biochemical and biophysical spatio-temporal cues that are necessary for improving organoid culture. Therefore, other matrices with defined compositions63 have been explored as alternative matrices to Matrigel, such as recombinant human collagen61, fibrin64 or synthetic hydrogels3,62. Natural matrices can be recombinantly produced from proteins or polysaccharides to address the batch to batch variability of Matrigel60. On the other hand, synthetic hydrogels have emerged as powerful tools that enable independent manipulation of biochemical and biophysical matrix properties to control organoid features and enhance functionality. The ideal organoid matrix should, overall, be stress-relaxing and highly dynamic in biochemical and biophysical properties to accommodate or control changes in organoid structure during culture. For example, dynamic hydrogels based on polyethylene glycol (PEG) were recently shown to enable reproducible intestinal organoid formation and demonstrate how hydrogel properties could be tuned to control stemness and differentiation in cultured organoids3. The viscoelastic profile of hydrogels has also been shown to define the mechanical confinement of growing organoids65. In other examples, the activity of adult stem cells can be controlled using PEG hydrogels with photo-degradable moieties66 and biomimetic polymers can be modified to incorporate essential ECM signals to generate organoids with tailored features3. Tunable PEG hydrogels can promote intestinal crypt budding67, whereas dextran-based GMP-compatible hydrogels support expansion of cells for longer passages68. Lastly, microfabricated arrays were recently reported to enable the uniform production of crypt–villi-shaped epithelium69. Even with the recent advancements using the synthetic matrix to grow organoids, organoid growth by the synthetic matrix is still less efficient than Matrigel-cultured organoids. There is an unmet demand to develop a better matrix.
尽管基质胶可以支持类器官培养,但这种生物来源的基质固有的异质性和定义不明确的组成几乎无法控制改善类器官培养所必需的生化和生物物理时空线索。因此,已经探索了具有特定成分的其他基质 63 作为基质的
Organoids can arise either from round colonies generated by single cells7,34 or from initial multicellular structures such as intestinal crypts29,62, cell aggregates23 or micropatterned cells25. The aim of the latter approach is to establish a cellular niche which involves other cells of the same or different type from the beginning. To form cell aggregates, the simplest method is to use an ultra-low-attachment dish coated with hydrophilic hydrogel70 to prevent cell attachment; subsequent centrifugation can promote aggregate formation, enhancing cell–cell contacts71. The size and compaction of cell aggregates can be tuned and controlled, such as through the use of microwell arrays71,72. Another well-established method to form cell aggregates is the hanging drop method, where aggregates form at the bottom of the drop. In a recent example, droplet microfluidics was used to aggregate murine cholangiocytes to form complex organoids with liver mesenchymal cells73. Droplet microfluidics can print one organoid per well and enable the rapid generation of intra-organoid heterogeneity74. Droplet-based microfluidics has also been used to perform better single-cell RNA sequencing (scRNA-seq) analysis of intestinal organoid cell identities during various developmental stages, revealing extensive population heterogeneity75. Microwell structures or microfabricated pillar arrays69 have also been developed to enable enhanced uniformity in cell aggregation71,72,76. In one example, microfabricated patterns of Laminin-512 were shown to reproducibly support human pluripotent stem cells to form lumen structures in Matrigel and differentiate into human neural tube-like structures25. These examples, amongst others emerging in the field, illustrate how our knowledge of biomaterials and tissue engineering can be extrapolated to provide precise control over organoid structure and function.
Soluble factors 可溶性因子
Organoid cultures are fundamentally based on our accumulated knowledge of developmental biology13, where soluble cues are presented to cells in a spatio-temporally controlled manner. Soluble factors used to differentiate TDCs and iPSCs into various tissue types are listed in Supplementary Table 1. In organoid culture, these soluble cues are recapitulated in vitro in the form of biologics, mainly as proteins such as growth factors77, or small-molecule drugs, which can activate or inhibit signalling pathways. Although growth factors could be costly and unstable, and many small-molecule drugs can affect multiple pathways resulting in poor reproducibility77, some organoid protocols have combined the use of both biologics and small-molecule drugs78. The use of conditioned medium from engineered cell lines producing biologically active growth factors, such as L-Wnt-3A, can replace commonly used growth factors, such as WNT3A ligands79. These conditioned media face a similar issue of batch to batch variation and require stringent tests to ensure reproducibility80. Thus, novel surrogate molecules are starting to arise as potential substitutes for conditioned medium79,80.
类器官培养从根本上基于我们积累的发育生物学知识 13,其中可溶性线索以时空控制的方式呈现给细胞。
It is critical to consider how and when soluble cues are added to organoid cultures because soluble cues in vivo are typically presented to cells by the ECM or nearby cells, coordinated in time and space. This is the concept of spatio-temporal presentation. For example, it is now known that fibroblast growth factor (FGF) activity and specificity can be regulated by cell-surface heparan sulfate proteoglycans, suggesting that the addition of free FGF into cell culture medium may not recapitulate how FGF is available in tissue in vivo81. The importance of presenting soluble cues in a spatio-temporally relevant manner is especially important for growing human iPSCs into complex structures with multiple cell lineages, such as in the case of kidney organoids82. Based on previous studies83, it is known that the ureteric epithelium develops from early migrating presomitic mesoderm cells. To recapitulate this process, the effect of different durations of initial Wnt signalling before the addition of FGF was investigated82. The spatio-temporal presentation of soluble factors can be achieved using different tissue engineering approaches. In one strategy, these growth factors can be encapsulated within nanoparticles, and conjugated onto cell surfaces for controlled release84,85,86,87. To mimic how certain growth factors are bound to the ECM in vivo, researchers conjugated polymers with heparin, which can bind to growth factors, or conjugated these growth factors to the polymer itself88. Surface tethering can also be achieved with nanotechnologies, such as nano-imprint lithography, electron beam and electrospinning, and substrates with nanopillars, nanopits or nanochannels, mimicking the basement membrane for 3D organoid culture89. Lastly, microfluidic systems can be leveraged to create miniaturized niches with precise control over mechano-chemical properties90. With directed flow and gradients of gas or small molecules, these systems can finely control environmental parameters within organoids17. In one example, a microfluidic neural tube device was developed to present simultaneous opposing gradients of growth factors to direct neural tube patterning, enabling recapitulation of the in vivo structure91.
Physical cues 物理线索
Beside biochemical cues, it is also important to consider when and whether it is necessary to provide appropriate physical cues to cultured organoids. Nutrient supply and waste removal, which are diffusion-dependent, become less efficient during organoid growth into larger tissue structures. This is the reason why intestinal organoids need to be fragmented into smaller cell clusters and re-seeded regularly7. Inadequate nutrient and waste diffusion is also problematic in brain organoid culture, where the resulting millimetre-sized constructs often exhibit necrosis within the inner core due to nutrient inaccessibility. This problem can be partially resolved using shaking cultures22,92, spinning bioreactors or suspension under continuous agitation22, or in continuously stirred bioreactors92. These bioreactors can monitor pH, temperature, oxygen and glucose levels to maximize mass transfer while minimizing shear stress. In this regard, perfusable microfluidic chips have also been developed to promote the long-term culture of organoids20,21,93. Lastly, it may be useful to consider providing topographical cues to control organoid culture in vitro; the topography of the substrate is known to modulate cell area, shape and cell–cell interactions, resulting in biochemical signals that can affect stem cell fate24. Topography-directed morphogenesis has been demonstrated using intestinal organoids grown on soft hydrogels94.
除了生化线索外,考虑何时以及是否有必要为培养的类器官提供适当的物理线索也很重要。依赖于扩散的营养供应和废物清除在类器官生长成更大的组织结构的过程中变得效率降低。这就是为什么肠道类器官需要被分解成更小的细胞簇并定期重新播种的原因 7。在脑类器官培养中,营养物质和废物扩散不足也是一个问题,由于营养物质无法获得,由此产生的毫米级构建体通常在内核内表现出坏死。这个问题可以使用摇动培养物 22,92,旋转生物反应器或在连续搅拌 22 下悬浮,或在连续搅拌的生物反应器 92 中部分解决。这些生物反应器可以监测 pH 值、温度、氧气和葡萄糖水平,以最大限度地提高传质效果,同时最大限度地减少剪切应力。在这方面,还开发了可灌注微流控芯片来促进类器官的长期培养 20,21,93。最后,考虑提供地形线索来控制体外类器官培养可能是有用的;已知底物的形貌可以调节细胞面积、形状和细胞间相互作用,从而产生可能影响干细胞命运的生化信号 24。使用在软水凝胶上生长的肠道类器官 94 证明了地形定向的形态发生。
Integrating cues 整合线索
In contrast to the original self-organizing organoid model, the above-described cues can be integrated to confer greater control over organoid morphogenesis. Integration of cues is a commonly employed strategy in the field of tissue engineering to construct tissues in vitro and in vivo95. Ideally, specific physical or chemical cues should be presented in a spatio-temporally, physiologically relevant manner using simple, reproducible and robust methods (Supplementary Table 2). We illustrate this using the example on intestinal organoids7. Following the identification and isolation of LGR+ intestinal stem cells residing in crypts, biologically derived Matrigel was leveraged to mimic the laminin-enriched crypt environment, supplemented with exogenously added growth factors in the cell culture medium. Most intestinal stem cells could survive, proliferate and form organoids7. However, given the stochastic nature of organoid development, the resulting organoids vary in size, bud number and function. In one example of how environmental cues (mechanical and biochemical) were integrated to exert control over organoid morphogenesis, the organoid maturation process was dissected into different stages, and biomaterials engineering was used to identify the optimal mechano-chemical environment at each stage3. It was shown that mechanically dynamic matrices that could switch from high to low stiffness over time enabled control over the morphogenesis process3. In another example, human neural tube morphogenesis25 was simulated using micropatterning, which created mechano-chemical gradients to regulate cell–cell/matrix interactions, and soluble factor presentation to orchestrate morphogenesis.
与原始的自组织类器官模型相比,可以整合上述线索以更好地控制类器官形态发生。线索的整合是组织工程领域在体外和体内构建组织的常用策略 95。理想情况下,应使用简单、可重复和稳健的方法以时空、生理相关的方式呈现特定的物理或化学线索(补充表 2)。我们用肠道类器官的例子来说明这一点 7.在鉴定和分离驻留在隐窝中的 LGR+ 肠道干细胞后,利用生物来源的基质胶来模拟富含层粘连蛋白的隐窝环境,并在细胞培养基中补充外源性添加的生长因子。大多数肠道干细胞可以存活、增殖并形成类器官 7.然而,鉴于类器官发育的随机性质,所得类器官的大小、芽数和功能各不相同。在如何整合环境线索(机械和生化)以控制类器官形态发生的一个例子中,类器官成熟过程被剖分为不同的阶段,并使用生物材料工程来确定每个阶段的最佳机械化学环境 3。研究表明,可以随时间从高刚度切换到低刚度的机械动态基质能够控制形态发生过程 3。 在另一个例子中,使用微图案模拟人类神经管形态发生 25,它创建了机械化学梯度来调节细胞-细胞/基质相互作用,并创建可溶性因子呈递来协调形态发生。
Besides these examples, other engineering methods have been used in organoid culture to control cell proliferation, differentiation and morphogenesis. A popular bioengineering approach for reconstructing tissues is bioprinting96 This technology uses bio-ink, comprising living organoids encapsulated within a biomaterial, to precisely create 3D biological geometries that mimic those of the native tissue in a layer by layer approach. In one recent example of how bioprinting was used to generate large tissue constructs, human small intestinal organoids were used to form self-evolving tissue constructs by a concept called bioprinting-assisted tissue emergence, where centimetre-scale intestinal tissues were printed sequentially to mimic boundaries in the gastrointestinal tract97. Another bioengineering approach to reconstruct tissues, especially across different tissues and organs, is the use of microfluidic platforms. Microfluidic systems have been used to create miniaturized cellular models comprising organoids that recapitulate critical aspects of organ physiology, known as the organ-on-a-chip98 (OoC). OoC devices can potentially incorporate other bioengineering techniques to imitate the key physiological and structural features of organs and tissues. For example, physical constraints were engineered into the organoid environment using synthetic scaffolds to provide physical boundaries99. OoC devices also support co-culture or multi-organ systems. The two-organ system has been simulated using connected chambers containing different tissue constructs to mimic liver injury (with intestinal epithelial cells and liver cells)100 and type 2 diabetes (with human pancreatic islets and liver spheroids)101. A two-organoid microfluidic device was recently fabricated with multiple read-outs using cardiac and hepatic organoids102. Moreover, OoC devices have improved the characteristics of human iPSC-derived pancreatic islets103, intestinal104, stomach105 and liver76 organoids by supporting physiological flow rates. Kidney organoids have been cultured in an OoC device that enables fluid flow to simulate shear stress106 and the generation of a vascular network107.
除了这些例子之外,类器官培养中还使用了其他工程方法来控制细胞增殖、分化和形态发生。重建组织的一种流行的生物工程方法是
Results 结果
It is important to evaluate whether cultured organoids recapitulate key aspects of the representative human tissue or organ. To achieve this, characterization of these organoids is typically performed by assessing organoid cellular composition, structure, functions and robustness of phenotype. In this section, we discuss commonly employed analytical methods using representative examples.
评估培养的类器官是否概括了代表性人体组织或器官的关键方面非常重要。为了实现这一目标,这些类器官的表征通常是通过评估类器官细胞组成、结构、功能和表型的稳健性来进行的。在本节中,我们将使用代表性示例讨论常用的分析方法。
Organoid composition and structure
类器官组成和结构
Organoid growth is a process of initial cell aggregation, proliferation, migration and differentiation. When assessing whether the organoids have been successfully established, it is critical to first determine whether the organoids contain the desired cell types, and the degree to which the organoids accurately mimic the functions of the corresponding tissue in vivo. To achieve this, both low-throughput gene expression validation and high-throughput whole-genome transcriptome analyses are typically performed. Real-time PCR is often first performed as an easy, fast and quantitative read-out on marker genes indicating cell identity, including key transcription factors and differentiation markers. Western blotting provides further quantitative information regarding protein abundance, protein degradation, protein–protein interactions and post-translational modifications, which represent the activities of a specific signalling pathway in a committed cell type. The most common tools used to evaluate organoid composition are immunofluorescence and immunohistochemical imaging using sections or whole mount, and the specific cell markers’ antibody staining further elucidates various cell types’ spatial distribution and proportion. A high-throughput analysis of scRNA-seq profiles all of the cell types in the organoids — undifferentiated and committed — at the whole-genome transcriptome level. These cell types are then compared with cells freshly isolated from the corresponding tissue or organ to evaluate the degree of similarity for each cell population. Given how it is expected that the in vitro cultured organoids contain different states of immature cells, scRNA-seq can be helpful for determining the extent of organoid heterogeneity in terms of cell differentiation status.
类器官生长是初始细胞聚集、增殖、迁移和分化的过程。在评估类器官是否成功建立时,首先
The assessment of organoid morphology is performed to determine whether there is structural similarity between the cultured organoids and corresponding in vivo tissue or organ108. For example, for secretory tissues such as pancreatic islets, the islet-like globular structure should be present within the organoids, and intra-cellular hormone vesicles109 are also expected to be observed. For branching epithelial organoids, such as breast organoids110, distinct branching structures are expected. For intestinal organoids, crypt-like structures99 should be observed. For more complex organoid culture systems comprising incorporated supportive cells, such as fibroblast111 and/or endothelial cells (ECs)106,109, it is expected that the incorporated ECs should form a vascular network106 that recapitulates the endothelial–epithelial interactions around the organoids.
进行类器官
Organoid functionality 类器官功能
Evaluation of organoid functionality includes, but is not limited to, the generation of mature cells, the formation of the vasculature or neuronal networks25,43, the accurate response to external stimuli and the effective secretion of cytokines or hormones, among other factors. Organoids should be compared with freshly isolated tissues as a positive control. Discrete physiological recapitulations are required for different tissues, such as acid secretion in gastric organoids and mucus secretion in intestinal organoids. Various methods of characterization to assess the structure and functions of organoids are listed in Table 1.
类器官功能的评估包括但不限于成熟细胞的产生、脉管系统或神经元网络的形成 25,43、对外部刺激的准确反应以及细胞因子或激素的有效分泌等因素。类器官应与新鲜分离的组织进行比较,作为阳性对照。不同组织需要离散的生理概括,例如胃类器官中的酸分泌和肠道类器官中的粘液分泌。表 1 列出了评估类器官结构和功能的各种表征方法。
表1 类器官研究评估/表征类器官结构和功能的方法
Pancreatic islet organoids
胰岛类器官
We use the example of mouse pancreatic islet organoids to discuss various characterization and validation methods (Fig. 3). The formation of four endocrine differentiated cell types (β-cell, α-cell, δ-cell and PP cell) is examined by the expressions of the corresponding hormones — insulin, glucagon, somatostatin and pancreatic polypeptide, respectively — by immunostaining112,113. To assess the extent of β-cell maturation, the ultrastructural morphometric analysis of insulin secretory granules is performed by immuno-gold transmission electron microscopy (TEM) imaging109. To evaluate the ability of the β-cells in organoid responsiveness to glucose, glucose-stimulated insulin secretion experiments are performed, for example using an insulin or a C-peptide ELISA assay with cyclic high and low glucose incubation114. As insulin release is associated with calcium dynamics, calcium signalling traces imaging rapidly increases in response to glucose and returns to baseline113,115. To test the full potential of the islet organoids upon transplantation, in vivo functional evaluation is needed to ameliorate the hyperglycaemic phenotypes of streptozotocin-induced type 1 diabetic mouse models109,113,116. The characterization parameters include normalized and stable blood glucose level, normal plasma insulin level and maintained body weight.
图3:胰岛类器官验证分析的代表性结果。
A, Representative view of pancreatic islet organoids at culture day 7. Aa, Organoids growing in Matrigel exhibit spherical shape. Ab, Viewed at high magnification, these spherical organoids are surrounded by endothelial cells (ECs) (arrows). Ac, A view of the organoids after they are released from Matrigel by dispase. B, Cell types and hormone secretion level validation by immunofluorescence or immunohistochemical staining. The expression of insulin (Ins; β-cells), glucagon (Gcg; α-cells), somatostatin (Sst; δ-cells) and pancreatic polypeptide (Ppy; PP cells) can be detected in mature organoids (shown by DAPI; blue). Scale bars, 20 μm. C, The morphology of mature organoids induced through prolonged culture for a total of 30 days: the organoids are surrounded by elaborate EC networks (parts Ca and Cb), shown by Cd31 (endothelium marker; red) and DAPI (organoid nuclei; blue) staining (part Cc). Scale bar, 50 μm. D, Real-time quantitative PCR analysis for some key transcription factors and differentiation markers. E, Pancreatic islet organoid function validation in vitro. Organoids are sequentially incubated with low (2 mM) and high (20 mM) concentration glucose for three rounds, followed by C-peptide ELISA and calcium imaging. F, Representative ELISA result of organoid secreting C-peptide measurement. The rise of C-peptide concentration in the media is sharply responsive to high glucose stimulation. **P < 0.01; ***P < 0.001. G, Representative curve of intensity of calcium signalling trace imaging, indicating the organoid’s capability of responding acutely to glucose, with high/low calcium activity consistent to high/low glucose stimulation. Potassium chloride incubation is used as a positive control. H, Islet organoid function validation in vivo. Organoids (or fresh islets as positive control) are transplanted into the kidney capsule of streptozotocin-induced diabetic mouse, followed by monitoring of certain physiological indexes, indicating the rescue of the diabetic phenotypes. Finally, the transplants are removed and the surging blood glucose levels in the mice are expected, further demonstrating that the transplants were responsible for the lowering glucose level effect. Parts A–C, F, G and H adapted from ref.113, Springer Nature Limited.
A,培养第 7 天胰岛类器官的代表性视图。Aa,在基质胶中生长的类器官呈球形。Ab,在高放大倍率下观察,这些球形类器官被内皮细胞(EC)包围(箭头)。Ac,类器官通过分散酶从基质胶中释放后的视图。B、通过免疫荧光或免疫组化染色验证细胞类型和激素分泌水平。胰岛素(Ins;β细胞)、胰高血糖素(Gcg;α细胞)、生长抑素(Sst;δ细胞)和胰多肽(Ppy;PP 细胞)可以在成熟类器官中检测到(由 DAPI 显示;蓝色)。比例尺,20 μm。C,通过延长培养总共 30 天诱导的成熟类器官的形态:类器官被复杂的 EC 网络( 部分 Ca 和 Cb)包围,由 Cd31(内皮标记;红色)和 DAPI(类器官核;蓝色)染色( 部分 Cc)显示。比例尺,50 μm。D、对一些关键转录因子和分化标志物进行实时荧光定量 PCR 分析。E,胰岛类器官体外功能验证。将类器官依次与低浓度(2mM)和高浓度(20mM)葡萄糖孵育三轮,然后进行 C 肽 ELISA 和钙成像。F,类器官分泌 C 肽测量的代表性 ELISA 结果。培养基中 C 肽浓度的升高对高葡萄糖刺激有急剧反应。 **P < 0.01;P < 0.001。 G,钙信号痕量成像强度的代表性曲线,表明类器官对葡萄糖的急性反应能力,高/低钙活性与高/低葡萄糖刺激一致。氯化钾孵育用作阳性对照。H,胰岛类器官体内功能验证。将类器官(或新鲜胰岛作为阳性对照)移植到链脲佐菌素诱导的糖尿病小鼠的肾包膜中,然后监测某些生理指标,表明糖尿病表型的挽救。最后,移除移植物,预计小鼠的血糖水平会飙升,进一步证明移植是降低血糖水平效应的原因。A-C、F、 G 和 H 部分改编自 Springer Nature Limited 的 ref.113。
Intestinal organoids 肠道类器官
Intestinal organoids are among the first organoid types successfully established in vitro7,29. Classical crypt–villus-like architecture is regarded as an important feature of successful organoid establishment, exhibiting proper branching morphology with robust multicellular composition and location reflecting the organoids’ differentiation and maturation level117,118. The expression of Lgr5 marker can analyse the maintenance of intestinal stem cells by real-time quantitative PCR or by imaging using a Lgr5-fluorescent reporter7,119. The differentiated cells are assessed by the staining of their markers, including lysozyme (for Paneth cells), villin (for enterocytes), Mucin 2 (for goblet cells) and chromogranin A (for enteroendocrine cells)7,99,120. These various cell types can also be distinguished by TEM according to their characteristic subcellular structure7,120. Functional intestinal organoids exhibit a relatively thick mucus layer due to mucus secretion from mature goblet cells121, which can be detected by Alcian blue and periodic acid–Schiff staining122,123.
肠道类器官是最早在体外成功建立的类器官类型之一 7,29。经典的隐窝-绒毛样结构被认为是成功建立类器官的重要特征,表现出适当的分支形态,具有强大的多细胞组成和位置,反映了类器官的分化和成熟水平 117,118。Lgr5 标记物的表达可以通过实时定量 PCR 或使用 Lgr5 荧光报告基因 7,119 进行成像来分析肠道干细胞的维持。通过染色来评估分化的细胞,包括溶菌酶(对于潘氏细胞),绒毛(对于肠细胞),粘蛋白 2(对于杯状细胞)和嗜铬粒蛋白 A(对于肠内分泌细胞)7,99,120。这些不同的细胞类型也可以通过 TEM 根据其特征性的亚细胞结构 7,120 来区分。由于成熟杯状细胞 121 的粘液分泌,功能性肠道类器官表现出相对较厚的粘液层,可以通过阿尔新蓝和高碘酸-希夫染色 122,123 检测到。
Liver organoids 肝脏类器官
In the case of the liver, both cholangiocyte34,35 and hepatocyte organoids124,125 have been established, both from mouse and human tissue samples. Measuring the passaging time and proliferation of organoid cells is used to assess liver organoids. Regarding marker expression, specific cholangiocyte (for example KRT19, KRT7, SOX9) or hepatocyte (such as ALB, HNF4A, MRP4) markers are assessed both on the RNA level (real-time quantitative PCR, scRNA-seq) and on the protein level (immunofluorescence staining). Often, also markers of liver progenitors (such as LGR5) are used to assess the differentiation state of the cultured cells. For the assessment of organoid functionality, further markers such as CYP3A4 and CYP3A11 for hepatocytes are analysed on the RNA level. Furthermore, albumin secretion, low-density lipoprotein uptake, the presence of glycogen accumulation assessed with periodic acid–Schiff staining, bile acid production and activities of liver enzymes such as CYP3A4 are assessed using chemical assays to confirm hepatocyte function. The ultrastructure of the organoids is also often assessed for typical hepatocyte or cholangiocyte morphology by TEM. Finally, for an ultimate test of functionality, organoids are often transplanted in FRG immune-deficient mice, to look at integration and functionality of the graft in vivo34,124,125.
在肝脏的情况下,胆管细胞 34,35 和肝细胞类器官 124,125 都已从小鼠和人体组织样本中建立。测量类器官细胞的传代时间和增殖用于评估肝脏类器官。
Validation of tumour organoids
肿瘤类器官的验证
Patient-derived tumour organoids must maintain the genomic, transcriptomic, morphologic and functional profiles of the tissue of origin. As such, it is important to validate them by comparison with the tissue they are derived from in terms of histology and immunohistochemistry profiles15, transcriptomics as well as genomics14,126. Cellular organization, tissue structure and protein expression patterns of tumour organoids can be easily compared with the cancer of origin15. Similarly, organoids are expected to have gene expression profiles similar to the tumour they are established from. This has been shown by RNA-seq on many tumour types including bladder cancer127 and oesophageal cancer128 amongst others. Lastly, it is important to confirm whether tumour organoids have diverged from the patient tumour at the genomic level. Several studies have shown concordance of organoids and parent tissue in terms of mutations and copy number variations14,126. A greater degree of divergence may be attributed to intra-tumour spatial heterogeneity and sampling bias: tumours can be spatially diverse, and organoids generated from a specific portion may lack the representativeness of distant regions129,130. In addition to this topographical issue, culture conditions can contribute to selecting specific clones, possibly altering or reducing clonality over time128.
患者来源的肿瘤类器官必须保持来源组织的基因组、转录组、形态学和功能特征。因此,重要的是通过与组织学和免疫组织化学特征 15,转录组学以及基因组学 14,126 方面与它们来源的组织进行比较来验证它们。肿瘤类器官的细胞组织、组织结构和蛋白质表达模式可以很容易地与起源癌症进行比较 15.同样,类器官应具有与它们所建立的肿瘤相似的基因表达谱。RNA-seq 已在许多肿瘤类型上表明了这一点,包括膀胱癌 127 和食管癌 128 等。最后,重要的是要确认肿瘤类器官是否在基因组水平上与患者肿瘤分化。几项研究表明类器官和亲本组织在突变和拷贝数变异方面具有一致性 14,126。更大程度的差异可能归因于肿瘤内空间异质性和抽样偏差:肿瘤可能在空间上是多样化的,并且从特定部分产生的类器官可能缺乏远处区域的代表性 129,130。除了这个地形问题之外,培养条件还有助于选择特定的克隆,随着时间的推移可能会改变或减少克隆性 128。
Validation of tumour organoids is even more critical when culturing over a long period of time. Whereas some studies have reported that molecular characteristics can be maintained over long-term culture, others have observed tumour evolution after serial passaging128,131. For instance, exome sequencing of liver cancer organoids showed that mutation concordance decreased from 92% for organoids cultured for less than 2 months to 80% for those grown for more than 4 months131. Colorectal tumour organoids established from microsatellite instable tumours had higher de novo mutations after long-term culture than stable ones58. Some of the differences observed may be attributable to how different clones and subclones adapt and expand in culture. Using deep targeted sequencing of 500 cancer-associated genes, truncal mutations were shown to be retained whereas subclonal ones are gained or lost, with mutational changes at each passage126. Most mutations and copy number variations are preserved in the late passage of lung cancer organoids, whereas de novo mutations can be attributed to the subclonal expansion of small subsets of cells present in the tumour of origin14. Overall, these studies showed that genetic drift can occur in organoids cultured for extended time, influencing their reliability as models for functional and drug discovery studies132. This reiterates the importance of validation in the context of tumour organoid establishment, characterization and testing. As an example of analysis, crucial steps in characterization of liver cancer organoids are demonstrated (Fig. 4). Lastly, normal tissue contamination is an issue for establishing tumour organoids and should be addressed when validating patient-derived models. The presence of normal cells can be estimated by histological comparison and immunohistochemistry, as well as sequencing54,133.
在长期培养时,肿瘤类器官的验证更为重要。虽然一些研究报告说分子特征可以在长期培养中保持,但其他研究则观察到连续传代后肿瘤的演变 128,131。例如,肝癌类器官的外显子组测序显示,突变一致性从培养少于 2 个月的类器官的 92%下降到生长超过 4 个月的类器官的 80%131。由微卫星不稳定肿瘤建立的结直肠肿瘤类器官在长期培养后比稳定肿瘤具有更高的新发突变 58。观察到的一些差异可能归因于不同的克隆和亚克隆如何在培养物中适应和扩展。使用对 500 个癌症相关基因进行深度靶向测序,躯干突变被保留,而亚克隆突变获得或丢失,每次传代都有突变变化 126。大多数突变和拷贝数变异在肺癌类器官的晚期传代中保留下来,而从头突变可归因于起源肿瘤中存在的小细胞子集的亚克隆扩增 14。总体而言,这些研究表明,遗传漂变可能发生在长时间培养的类器官中,从而影响其作为功能和药物发现研究模型的可靠性 132。这重申了在肿瘤类器官建立、表征和测试背景下验证的重要性。 作为分析的一个例子,展示了肝癌类器官表征的关键步骤(图 4)。最后,正常组织污染是建立肿瘤类器官的一个问题,在验证患者来源的模型时应解决。正常细胞的存在可以通过组织学比较和免疫组织化学以及测序 54,133 来估计。
图 4:癌症类器官的典型特征:肝癌亚型 131。
a, Isolation of cells from patient samples and organoid culture, with schematic of tissue isolation and processing. b, Histological analysis of liver cancer samples. Top row shows tissue (scale bar: 50 μm), middle row shows organoid bright-field images (scale bar: 100 μm) and bottom two rows show histological haematoxylin and eosin (H&E) staining of organoids (scale bar: 50 μm). c, Analysis of specific marker gene expression, including immunofluorescence staining for AFP (hepatocyte/hepatocellular carcinoma (HCC) marker; red) and EpCAM (ductal/cholangiocarcinoma (CC) marker; green). DAPI staining shown in blue. Scale bar: 30 µm. d, Organoid passaging shown as growth and splitting curves, where dots represent a splitting time point and arrows represent continuous expansion. e, Transplantation of xenografts into immunodeficient mice. Xenograft and histopathology analysis, matching the patient’s original tissue sample. Scale bars: 125 μm, inset: 62.5 μm. f, Analysis of genetic changes in the cancer organoids and their concordance to the mutations in the original tumour sample. g, Half-maximal inhibitory concentration (IC50) curves for gemcitabine treatment showing organoid sensitivity to drugs. CHC, combined HCC/CC tumours; PLC, primary liver cancer; SC, subcutaneous. Adapted from ref.131, Springer Nature Limited.
a,从患者样本和类器官培养中分离细胞,并附有组织分离和处理示意图。b、肝癌样本的组织学分析。上行显示组织(比例尺:50 μm),中行显示类器官明场图像(比例尺:100 μm),下两行显示类器官的组织学苏木精和伊红(H&E)染色(比例尺:50 μm)。c,分析特定标记基因表达,包括 AFP(肝细胞/肝细胞癌(HCC)标志物;红色)和 EpCAM(导管/胆管癌(CC)标志物;绿色)的免疫荧光染色。DAPI 染色以蓝色显示。比例尺:30μm。d,类器官传代显示为生长和分裂曲线,其中点代表分裂时间点,箭头代表连续扩增。e,将异种移植物移植到免疫缺陷小鼠体内。异种移植物和组织病理学分析,匹配患者的原始组织样本。比例尺:125 μm,插图:62.5 μm。f,分析癌症类器官的遗传变化及其与原始肿瘤样本中突变的一致性。g,吉西他滨治疗的半最大抑制浓度 (IC50) 曲线显示类器官对药物的敏感性。CHC,合并 HCC/CC 肿瘤;PLC,原发性肝癌;SC,皮下注射。改编自 Springer Nature Limited 的 ref.131。
Data analysis 数据分析
Image processing and analyses
图像处理和分析
ImageJ/Fiji134, CellProfiler135 and other software are used for image processing. The number of organoids formed in a certain period, organoid size (measured as area or volume) and cell number can measure the cell proliferation rate. Cell proliferation can be quantified by the ratio of EdU-positive or Ki67-positive nuclei using available software plug-ins. Morphological evaluation — such as the bud formation ratio in the intestine organoid system — is also necessary when quantifying the success rate considering functional maturation. Mean and/or normalized fluorescence intensity of maturation markers for different tissue types can be recorded to measure the organoid maturation level. Long-term live imaging can be analysed using software to track the cell behaviour for lineage tracing purposes. Web resources are used for pathway analysis and visualization of multi-omics data to generate heat maps for understanding the differentiation stage of the organoid136,137. Other software, such as Profiler, GSEA, Cytoscape and EnrichmentMap, have been used for biomolecular interaction networks, pathway enrichment analysis and visualization138,139,140,141.
图像处理采用 ImageJ/Fiji134、CellProfiler135 等软件。在一定时期内形成的类器官数量、类器官大小(以面积或体积测量)和细胞数量可以测量细胞
Data analytics classification of phenotypes
表型的数据分析分类
Miniaturization and automation using machine learning and artificial intelligence-based data analytics make high-throughput and multidimensional phenotyping of organoids more consistent. Various software for these purposes have been developed in recent years, including ilastik142, OrganoidTracer143 and Phindr3D (ref.144). Most of these have Fiji or CellProfiler plug-ins, which make handling large data sets in shorter time frames possible for users without substantial computational expertise. Those software usually contain functions for image segmentation, object classification, morphological characterization, fluorescence intensity quantification, cell counting and tracking for hundreds of images. Good image quality is a prerequisite for a robust result, so optimizing the image acquisition process is crucial. At different stages of the data processing, validation of the accuracy of the trained algorithm is recommended.
使用机器学习和基于人工智能的数据分析的小型化和自动化使类器官的高通量和多维表型更加一致。近年来,已经开发了用于这些目的的各种软件,包括 ilastik142、OrganoidTracer143 和 Phindr3D(参考文献 144)。其中大多数都有 Fiji 或 CellProfiler 插件,这使得没有大量计算专业知识的用户可以在更短的时间内处理大型数据集。这些软件通常包含图像分割、对象分类、形态学表征、荧光强度量化、细胞计数和数百张图像跟踪的功能。良好的图像质量是获得稳健结果的先决条件,因此优化图像采集过程至关重要。在数据处理的不同阶段,建议验证训练算法的准确性。
Applications 应用
Organoids as engineered cell-based models to recapitulate relevant physiological structures and functions of interest have shown great potential in both basic research and clinical applications. Here, we summarize a few applications.
类器官作为基于工程细胞的模型,用于概括感兴趣的相关生理结构和功能,在基础研究和临床应用中都显示出巨大的潜力。在这里,我们总结一些应用。
Tissue regeneration 组织再生
Tissue-derived organoids can be a potential source of transplantable material for regenerative medicine. Organoids of murine intestines30, livers34,124,125 and pancreas68,145 have been successfully transplanted into mice with restoration of organ function. For example, liver organoids have been generated from hepatocytes isolated from murine livers125. The organoids were injected into mice with a deficiency in fumarylacetoacetate hydrolase (Fah), a defect causing liver injury resulting in short survival (40 days) after treatment with the hepato-protective nitisinone drug is withdrawn34. Organoid engraftment rates were as high as 80%, and the engrafted mice survived longer than 100 days after the interruption of nitisinone therapy125. Similarly, pancreatic islets cultured in an artificial ECM could be successfully engrafted into either streptozocin-induced or autoimmune-driven diabetic mouse models, rescuing insulin production and reversing hyperglycaemia146. Beyond murine organoids, human liver organoids derived from EpCAM+ ductal cells from liver samples have been engrafted into immunodeficient mice with acute liver damage induced34. Human pancreas organoids can be generated from ALDHhigh stem cells and have the potential to produce insulin145 without in vivo evidence yet to confirm engraftment. Intestinal organoids have been shown to fuse into tubular structures resembling in vivo intestines147,148. A more complicated intestinal organoid can also be generated in an artificial ECM with modulable biodegradability3. Although there is a long way to go for organoids to be used in regenerative medicine, improved protocols to generate normal tissue organoids, good manufacturing practices ensuring high reproducibility, grafting and function can already be established149.
组织来源的类器官可以成为再生医学可移植材料的潜在来源。小鼠肠道 30、肝脏 34,124,125 和胰腺 68,145 的类器官已成功移植到小鼠体内,器官功能恢复。例如,肝脏类器官是从小鼠肝脏中分离的肝细胞产生的 125。将类器官注射到缺乏富马酰乙酰乙酸水解酶 (Fah) 的小鼠体内,这种缺陷会导致肝损伤,导致用保肝尼替西农药物治疗后生存期短(40 天)34。类器官 植入率高达 80%,植入小鼠在尼替西农治疗中断后存活超过 100 天 125。同样,在人工 ECM 中培养的胰岛可以成功地移植到链脲佐星诱导或自身免疫驱动的糖尿病小鼠模型中,从而挽救胰岛素的产生并逆转高血糖 146。除了小鼠类器官之外,源自肝脏样本中 EpCAM+ 导管细胞的人肝类器官已被植入诱导急性肝损伤的免疫缺陷小鼠体内 34。人胰腺类器官可以由 ALDH 高干细胞产生,并有可能产生胰岛素 145,但体内证据尚未证实植入。肠道类器官已被证明可以融合成类似于体内肠道的管状结构 147,148。 在具有可调节生物降解性的人工 ECM 中也可以产生更复杂的肠道类器官 3。尽管类器官用于再生医学还有很长的路要走,但已经可以建立生成正常组织类器官的改进方案、确保高可重复性、移植和功能的良好生产规范 149。
Drug discovery and development studies
药物发现和开发研究
Technical challenges to rapid and high-throughput screening of 3D organoids have been addressed in recent years and surpassed by different means, including alternative plate design or seeding geometries15,150,151. A screening of 240 tyrosine kinase inhibitors on organoids derived from patients with ovarian or peritoneal tumours provided individualized drug sensitivity profiles within a week from surgery. The study included a carcinosarcoma of the ovary, a sporadic ovarian cancer for which no line therapy was established150. Tumour organoids can be screened to select effective drugs from structurally similar candidates152 and investigate the synergic effects of different drugs for combination therapy153. Non-tumour cells from adjacent normal tissue in biopsy samples or surgically resected samples can be used to develop control healthy organoids to confirm the tumour-specific efficacy of the tested drugs154. Organoids can be screened with libraries of immune cells engineered to express chimeric antigen receptors against different neo-antigens155. Checkpoint inhibitors and other immuno-oncology drugs can also be screened in tumour organoid models that include immune cells156.
近年来,3D 类器官快速和高通量筛选的技术挑战已经得到解决,并通过不同的方式克服,包括替代板设计或接种几何形状 15,150,151。对卵巢或腹膜肿瘤患者来源的类器官的 240 种酪氨酸激酶抑制剂的筛选提供了手术后一周内的个体化药物敏感性特征。该研究包括卵巢癌肉瘤,这是一种散发性卵巢癌,尚未建立线治疗 150。可以筛选肿瘤类器官以从结构相似的候选药物 152 中选择有效的药物,并研究不同药物的协同作用用于联合治疗 153。活检样本或手术切除样本中来自相邻正常组织的非肿瘤细胞可用于开发对照健康类器官,以确认测试药物的肿瘤特异性疗效 154。类器官可以通过免疫细胞文库进行筛选,这些文库被改造为表达针对不同新抗原的嵌合抗原受体 155。检查点抑制剂和其他免疫肿瘤药物也可以在包括免疫细胞的肿瘤类器官模型中筛选 156。
Biomarker research 生物标志物研究
As organoids can maintain the genomic profile of the parent tissue157, drug screening can be used to provide connections between genetic mutations and drug responses36,153,158. By screening gastric cancer organoids from 7 patients against 37 drugs, tumours with ARID1A mutations were shown to be sensitive to ATR inhibitors158, supporting a previous study159 and functionally identifying an intervention for a class of mutations that were considered undruggable.
由于类器官可以维持亲本组织的基因组图谱 157,因此药物筛选可用于提供基因突变和药物反应之间的联系 36,153,158。通过对 7 名患者的胃癌类器官进行 37 种药物的筛选,具有 ARID1A 突变的肿瘤被证明对 ATR 抑制剂敏感 158,支持之前的研究 159,并在功能上确定了针对一类被认为不可成药的突变的干预措施。
Precision medicine applications
精准医疗应用
There is growing interest in tailoring cancer therapy to each patient. Precision medicine is often synonymous with genomics160. However, as shown in several clinical trials160,161,162,163,164, only a small proportion of patients have an actionable alteration that can be coupled to a specific intervention160. In the NCI-MATCH trial that evaluates broad-scale implementation of sequencing-driven therapy, only approximately 37% of patients were found to have an actionable mutation165. This and other precision medicine trials have shown limited clinical benefits and modest response rates for many of the targeted interventions, uncovering a large degree of complexity165,166,167. For instance, the SHIVA trial that compared the outcome of genomic-guided interventions with conventional therapy selected by the oncologist for heavily pretreated patients with cancer showed no difference in progression-free survival160,161. Although the clinical efficacy of genomics-based precision medicine interventions can be debated, it is clear that most patients with cancer do not have a targetable genetic to guide therapy. Functional precision medicine — based on testing drug responses on patient tumours to identify treatment regimens — has been proposed as a more direct alternative, as it does not require any a priori knowledge of the tumour molecular profile or other characteristics160,168. Organoids are ideally suited for functional precision medicine because they are straightforward to establish, have high similarity with the tissue of origin, are tractable157,169 and have been deployed to identify therapeutic leads in numerous tumours170, including rare cancers that tend to be understudied and poorly characterized at the molecular level15,150,171,172. Recently, organoids established from patient-derived xenografts were used to identify eribulin as therapy for a patient with recurrent triple-negative breast cancer173. The patient’s progression-free survival was 3.5× longer on an eribulin regimen than on the previous therapy received173, indicating the potential of organoids in clinical care.
人们对为每位患者量身定制癌症治疗越来越感兴趣。精准医学通常是基因组学的代名词 160。然而,正如几项临床试验 160,161,162,163,164 所示,只有一小部分患者具有可作的改变,可以与特定干预措施相结合 160。在评估测序驱动疗法的广泛实施的 NCI-MATCH 试验中,只有大约 37% 的患者被发现具有可作的突变 165。这项试验和其他精准医学试验显示,许多有针对性的干预措施的临床获益有限,反应率适中,揭示了很大程度的复杂性 165,166,167。例如,SHIVA 试验将基因组引导干预的结果与肿瘤学家为经过大量预处理的癌症患者选择的常规疗法进行了比较,结果显示无进展生存期没有差异 160,161。尽管基于基因组学的精准医学干预的临床疗效可以争论,但很明显,大多数癌症患者没有可靶向的基因来指导治疗。功能性精准医学——基于测试患者肿瘤的药物反应以确定治疗方案——已被提议作为一种更直接的替代方案,因为它不需要对肿瘤分子谱或其他特征进行任何先验知识 160,168。 类器官非常适合功能性精准医学,因为它们易于建立,与起源组织具有高度相似性,易于处理 157,169,并已被用于识别许多肿瘤中的治疗先导化合物 170,包括在分子水平上往往研究不足且特征不佳的罕见癌症 15,150,171,172. 最近,从患者来源的异种移植物中建立的类器官被用于确定艾日布林作为复发性三阴性乳腺癌患者的治疗 173。艾日布林方案患者的无进展生存期比之前接受的治疗长 3.5× 173,表明类器官在临床护理中的潜力。
Source material for xenografts
异种移植物的来源材料
Organoids are increasingly being used as seeding material to generate xenografts with engraftment rates. This process involves using intact organoids14,58 or individual cells after digestion54,174. Human intestinal organoids transformed by CRISPR–Cas9 to carry mutations in APC, SMAD4, TP53, KRAS and/or PIK3CA were seeded into the subcapsular kidney space of immunocompromised mice. Benign organoids carrying mutations in one or two genes were shown as unable to form tumours, whereas organoids with mutations in four or five genes were tumorigenic175.
类器官越来越多地被用作种子材料,以产生具有植入率的异种移植物。该过程涉及使用完整的类器官 14,58 或消化后的单个细胞 54,174。将 CRISPR-Cas9 转化的人肠道类器官携带 APC,SMAD4,TP53,KRAS 和/或 PIK3CA 突变,接种到免疫功能低下小鼠的包膜下肾腔中。 携带一个或两个基因突变的良性类器官被证明无法形成肿瘤,而具有四个或五个基因突变的类器官是致瘤的 175。
Patient-derived organoids can also generate xenografts, confirm their tumorigenic capacity and recapitulate the histology of parental tumours14,54. In a study of colorectal cancer, benign tumour organoids showed no or minimal engraftment in mice. By contrast, colorectal cancer organoids derived from metastases were more invasive than those from primary tumours58. Comparison of the morphology of xenografts formed by glioblastoma multiforme cells cultured in vitro either as tumour spheres or organoids showed that whereas cells from tumorspheres displayed a solid growth pattern, organoids were more diffusive, similar to the tumour of origin174. Organoid lines derived from bladder tumours of lumenal origin transplanted in mice were shown to give rise to tumours with the same lumenal phenotype126.
患者来源的类器官还可以产生异种移植物,确认其致瘤能力并概括亲本肿瘤的组织学 14,54。在一项针对结直肠癌的研究中,良性肿瘤类器官在小鼠中没有或很少有植入。相比之下,源自转移灶的结直肠癌类器官比源自原发肿瘤的类器官更具侵袭性 58。比较由多形性胶质母细胞细胞形成的异种移植物的形态,无论是作为肿瘤球体还是类器官,都表明,虽然来自肿瘤球的细胞表现出固体生长模式,但类器官更具扩散性,类似于起源肿瘤 174。源自移植到小鼠体内的管腔来源的膀胱肿瘤的类器官系被证明会产生具有相同管腔表型的肿瘤 126。
Infectious diseases 传染病
Organoid models are now widely used in research on host–pathogen interactions. For instance, organoids derived from tissues and organs such as the intestine176,177, liver178, lung177,179, oral mucosa180 and stomach32,181 have been co-cultured with pathogens such as bacteria32,181,182, viruses176,178,179,180,183 and parasites177. In human intestinal organoids, differentiated intestinal cells are more susceptible to human rotavirus (HRV) infection than undifferentiated ones. Organoids infected with HRV or rotavirus enterotoxin displayed lumenal swelling, a characteristic of rotavirus-induced diarrhoea183. Human liver organoid was used as a drug screening platform to test anti-HRV drugs and antibodies, identifying mycophenolic acid, IFNγ and anti-rotavirus VP7 antibodies capable of blocking rotavirus replication ex vivo178. Organoids can also be used in studies of oncogenic pathogens. Infection of murine gastric organoids by Helicobacter pylori showed increased cytosolic β-catenin levels induced by bacterially originated CagA, which led to increased proliferation in infected organoids181.
类器官模型现在广泛用于宿主-病原体相互作用的研究。例如,源自肠道 176,177、肝脏 178、肺 177,179、口腔粘膜 180 和胃 32,181 等组织和器官的类器官已与细菌 32,181,182、病毒 176,178,179,180,183 和寄生虫 177.在人肠道类器官中,分化的肠道细胞比未分化的肠道细胞更容易受到人轮状病毒 (HRV) 感染。感染 HRV 或轮状病毒肠毒素的类器官表现出管腔肿胀,这是轮状病毒诱导的腹泻的特征 183。以人肝类器官为药物筛选平台,检测抗 HRV 药物和抗体,鉴定出能够阻断轮状病毒离体复制的麦考酚酸、IFNγ和抗轮状病毒 VP7 抗体 178。类器官也可用于致癌病原体的研究。 幽门螺杆菌对小鼠胃类器官的感染显示出由细菌来源的 CagA 诱导的胞质β-连环蛋白水平增加,这导致受感染类器官的增殖增加 181。
Since the start of the COVID-19 pandemic, several laboratories have investigated the effects of the SARS-CoV-2 virus on organoids. SARS-CoV-2-infectable distal lung organoids were generated with ACE2-expressing lumenal cells184. SARS-CoV-2 was found to target goblet cells rather than ciliate ones, in contrast to a previous study in a 2D air–liquid interface model185. Studies in intestinal organoids show that SARS-CoV-2 can also infect enterocytes185,186, and CRISPR–Cas9 and drug screenings have shown that knock-outs of either TMPRSS2 or TMPRSS4, or treatment with Camostat, can reduce viral infection186.
自 COVID-19 大流行开始以来,一些实验室已经研究了 SARS-CoV-2 病毒对类器官的影响。用表达 ACE2 的管腔细胞生成 SARS-CoV-2 感染的远端肺类器官 184。发现 SARS-CoV-2 靶向杯状细胞而不是纤毛细胞,这与之前在 2D 气液界面模型 185 中的一项研究形成鲜明对比。肠道类器官研究表明,SARS-CoV-2 也可以感染肠细胞 185,186,CRISPR-Cas9 和药物筛选表明,敲除 TMPRSS2 或 TMPRSS4,或用 Camostat 治疗,可以减少病毒感染 186。
Biology of common and rare diseases
常见病和罕见病的生物学
Organoids have demonstrated significant utility in modelling and investigating both common as well as rare diseases arising from various different organs. Cystic fibrosis is a genetic disorder caused by mutations in the gene CFTR, which expresses a transmembrane chloride and bicarbonate transporter. More than 2,000 mutations in CFTR associated with cystic fibrosis have been reported, yet small-molecule therapeutics deployed clinically only target a subset of these187,188. Mutations in CFTR cause multi-organ dysfunctions affecting the lungs, pancreas, liver and intestine189. Cystic fibrosis organoid models of the colon190, liver and bile ducts191, rectum192,193, the respiratory system179,194 and the small intestine190,195 have been derived from adult stem cells of relevant organs of either mouse models or patients, with applications ranging from investigating the biology of disease to mirroring patients’ drug responses192. Forskolin, a CFTR activator, has been used to induce organoid swelling due to chloride and fluid flux into the lumen193, with reduced swelling of patient-derived rectal organoids correlated with clinical response parameters of patients, which could mimic individualized clinical responses to specific therapeutic regimens193. Cystic fibrosis organoids have also been used to investigate gene therapy interventions and are able to rescue the effect of CFTR mutations in intestinal organoids using CRISPR–Cas9 (ref.195).
类器官在模拟和研究由各种不同器官引起的常见和罕见疾病方面已显示出显着的效用。囊性纤维化是一种由表达跨膜氯化物和碳酸氢盐转运蛋白的 CFTR 基因突变引起的遗传性疾病。据报道,与囊性纤维化相关的 CFTR 突变有 2,000 多种,但临床上部署的小分子疗法仅针对这 187,188 种中的一部分。CFTR 突变会导致影响肺、胰腺、肝脏和肠道的多器官功能障碍 189。结肠 190、肝脏和胆管 191、直肠 192,193、呼吸系统 179,194 和小肠 190,195 的囊性纤维化类器官模型已来源于小鼠模型或患者相关器官的成体干细胞,其应用范围从研究疾病生物学到反映患者的药物反应 192. 毛喉素是一种 CFTR 激活剂,已被用于诱导由于氯化物和液体通量进入管腔而引起的类器官肿胀 193,患者来源的直肠类器官肿胀减少与患者的临床反应参数相关,这可以模拟对特定治疗方案的个体化临床反应 193.囊性纤维化类器官也已用于研究基因治疗干预,并能够使用 CRISPR-Cas9 挽救肠道类器官中 CFTR 突变的影响(参考文献 195)。
Inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease have also been studied with organoid models196. Organoids derived from intestinal epithelial cells of paediatric patients with inflammatory bowel diseases had inflammatory bowel disease-specific DNA methylation signatures despite retaining similar morphologies197. Liver organoids were generated to model liver diseases such as α1-antitrypsin deficiency35, Alagille syndrome35 and primary sclerosing cholangitis198. Lung organoids have been established to investigate diseases such as goblet cell metaplasia199.
溃疡性结肠炎和克罗恩病等炎症性肠病也已使用类器官模型进行了研究 196。源自炎症性肠病儿科患者肠上皮细胞的类器官尽管保留了相似的形态,但仍具有炎症性肠病特异性 DNA 甲基化特征 197。生成肝脏类器官来模拟肝脏疾病,例如 α1-抗胰蛋白酶缺乏症 35、Alagille 综合征 35 和原发性硬化性胆管炎 198。肺类器官已被建立用于研究杯状细胞化生等疾病 199。
Reproducibility and data deposition
可重复性和数据沉积
Heterogeneity and reproducibility
异质性和可重复性
Organoids exhibit heterogeneity and variability between cells forming the organoid (intra-organoid heterogeneity), between organoids in the same dish and between individual patients (inter-organoid heterogeneity). This heterogeneity is valuable to recapitulate the individual to individual differences, especially in the context of human diseases, and is valuable in cancer research to mimic patient-specific cancer development for personalized medicine126,127,128,131,154,158,169,200,201,202. Similarly, the intra-organoid variation reflects the complexity of cellular composition of the tissues. This is advantageous for modelling tissue development or regeneration, with cells forming an organoid encompassing different cellular states, from stem/progenitor cells to more differentiated cells35,124,203. Although these levels of variation and heterogeneity reflect the complexity of biological systems, they can jeopardize the reproducibility and robustness of the system when left uncontrolled.
类器官在形成类器官的细胞之间表现出异质性和变异性(类器官内异质性)、同一培养皿中的类器官之间以及个体患者之间(类器官间异质性)。这种异质性对于将个体概括为个体差异很有价值,特别是在人类疾病的背景下,并且在癌症研究中很有价值,以模拟患者特异性癌症发展以进行个性化医疗 126,127,128,131,154,158,169,200,201,202.同样,类器官内的变化反映了组织细胞组成的复杂性。这对于模拟组织发育或再生是有利的,细胞形成的类器官包含不同的细胞状态,从干细胞/祖细胞到更分化的细胞 35,124,203。尽管这些程度的变异和异质性反映了生物系统的复杂性,但如果不加以控制,它们可能会危及系统的可重复性和稳健性。
Significant differences in morphology and functionality or even organoid formation efficiency depend on the different cells of origin of the culture or different cells in the organoid, as well as different medium composition119,204,205,206. Regarding the cells of origin, the batch and quality of the iPSCs/pluripotent stem cells used to generate the starting culture can influence the variability of the organoid line obtained. Similarly, the different primary cells isolated from different animals or patients also impact the subsequent cultures12. The media are usually optimized for cell proliferation whereas differentiation towards certain cell fates can be largely influenced by the culture conditions, thus increasing variability and heterogeneity. Refining of culture conditions has been demonstrated to increase the diversity of differentiated cell fates in human small intestinal organoids119. Undefined ECMs (such as Matrigel) have unknown composition and batch to batch variability, making reproducibility a significant concern12,207. Another important factor becomes apparent when looking into transcriptomic changes during organoid development208 or studying gene expression changes during organoid passages34,204. When organoids mimic development, these changes can lead to confounding effects, for example, the temporal heterogeneity. Therefore, recording the passage and the time in culture is essential to identify sources of variation that affect the results.
形态和功能甚至类器官形成效率的显着差异取决于培养物来源细胞的不同或类器官中的不同细胞,以及不同的培养基组成 119,204,205,206。关于来源细胞,用于产生起始培养物的 iPSC /多能干细胞的批次和质量会影响所获得的类器官系的变异性。类似地,从不同动物或患者中分离出的不同原代细胞也会影响后续培养物 12。培养基通常针对细胞增殖进行优化,而向某些细胞命运的分化可能在很大程度上受到培养条件的影响,从而增加变异性和异质性。培养条件的改进已被证明可以增加人小肠类器官中分化细胞命运的多样性 119。未定义的 ECM(例如 Matrigel)具有未知的组成和批次间变异性,这使得可重复性成为一个重要问题 12,207。在研究类器官发育过程中的转录组变化 208 或研究类器官传代过程中的基因表达变化时,另一个重要因素变得明显 34,204。当类器官模拟发育时,这些变化会导致混杂效应,例如时间 异质性 。因此,记录培养物中的传代和时间对于确定影响结果的变异源至关重要。
To better understand and control heterogeneity, the field has been moving from bulk analysis towards employing single-cell approaches, such as scRNA-seq, to help delineate the different populations of organoid-forming cells. Many single cell-resolved data sets are emerging, with several notable examples in more homogeneous organoid production3,69,99,209 and resolving organoid heterogeneity during growth progression206,210. Pooling together different cells and/or organoids for bulk analysis can lead to inaccurate results3,69,99,206,209,210. Therefore, good record-keeping about what was pooled in the experiment deems crucial to drawing robust conclusions.
为了更好地理解和控制异质性,该领域一直在从批量分析转向采用单细胞方法,例如 scRNA-seq,以帮助描绘类器官形成细胞的不同群体。许多单细胞解析数据集正在出现,在更均匀的类器官生产 3,69,99,209 和解决生长过程中的类器官异质性方面有几个值得注意的例子 206,210。将不同的细胞和/或类器官合并在一起进行批量分析可能会导致结果不准确 3,69,99,206,209,210。因此,对实验中汇集的内容进行良好的记录对于得出可靠的结论至关重要。
Additionally, it is critical to reduce the sources of variability and heterogeneity as the field grows in complexity. Efforts to generate more complete organoid structures containing different cellular populations (reviewed elsewhere211,212) — such as the recently published endothelial and healthy human colon and human colorectal cancer organoids213, murine cholangiocyte and mesenchymal organoids73 and murine pancreatic islet and endothelial organoids109 — will require controlling sources of variation derived from incorporating additional cells to an already heterogeneous system. Exerting control over the variation while generating more functionally relevant structures represents a significant challenge for the field.
此外,随着该领域的复杂性增加,减少变异性和异质性的来源也至关重要。努力产生包含不同细胞群的更完整的类器官结构(在其他地方评论 211,212)——例如最近发表的内皮和健康人结肠癌和人结直肠癌类器官 213、小鼠胆管细胞和间充质类器官 73 以及小鼠胰岛和内皮类器官 109——将需要控制因将额外的细胞合并到已经异质的系统中而产生的变异源。在生成功能更相关的结构的同时控制变异对该领域来说是一个重大挑战。
Finally, significant heterogeneity is also observed following organoid treatments. Although this represents a drawback when trying to use organoids as a tool for investigating molecular mechanism, it is beneficial when aiming to model patient responses. In particular, for cancer treatment, several different patient-derived organoid models tested (including, but not limited to, pancreas, colorectal and liver cancer) exhibit heterogeneous responses to known chemotherapeutic and anticancer agents48,131,214. This parallels the variability in patient outcomes observed in the clinic and has positioned cancer organoids as a potential predictive tool for drug testing (reviewed elsewhere215). This heterogeneity in treatment responses is now being exploited for the treatment of patients with cystic fibrosis193, with a large ongoing European multicentre clinical trial study, led by the HIT-Cystic Fibrosis consortium, to identify potential responders216. Similar initiatives are lagging behind for patients with cancer, although mostly because of disparities in establishment rates and lengthy timelines (reviewed elsewhere217). Organoids were recently used to inform the treatment of a patient suffering from a rare form of liver cancer, although unfortunately the treatment was discontinued due to deterioration of the patient’s general condition, preventing the authors from drawing conclusions regarding the predictive value of the organoid system for drug treatment218.
最后,在类器官处理后也观察到显着的异质性。尽管这在尝试使用类器官作为研究分子机制的工具时是一个缺点,但在旨在模拟患者反应时它是有益的。特别是,对于癌症治疗,测试的几种不同的患者来源的类器官模型(包括但不限于胰腺癌、结直肠癌和肝癌)对已知的化疗和抗癌药物表现出异质反应 48,131,214。这与临床上观察到的患者预后的变异性相似,并将癌症类器官定位为药物测试的潜在预测工具( 详见别处 215)。这种治疗反应的异质性现在被用于治疗囊性纤维化患者 193,由 HIT-囊性纤维化联盟领导的一项正在进行的大型欧洲多中心临床试验研究,以确定潜在的反应者 216。对于癌症患者来说,类似的举措落后了,尽管主要是因为建立率的差异和漫长的时间表(在其他地方回顾了 217)。类器官最近被用于为患有罕见肝癌的患者提供信息,尽管不幸的是,由于患者一般状况恶化,治疗被停止,使作者无法得出关于类器官系统对药物治疗的预测价值的结论 218。
Minimum reporting standards
最低报告标准
To improve the reproducibility and reliability of the results, it is essential to account for the variability between different cell isolations and intra-organoid heterogeneity. Although one could consider that at least three independent experiments with at least three different biological replicates are a minimum requirement, this may not always be sufficient. In fact, the number of organoids to study per biological replicate as well as the number of biological replicates depends on the research question, the specific experiment and the variability of the phenotype observed. Whenever possible, calculating the number of independent organoids per biological replicate, sample size calculations similar to those used for mouse experiments, taking into account statistical power and variance of the experiment, would be highly recommended. The data should be robust, meaning repeated in at least three independent biological replicates, which refers to independent isolations from different animals or patients. However, replicates and the research question for each experiment should be determined in advance to avoid hypothesis fishing and increasing replicates to get a significance between conditions219,220. Data should be reported correctly as a mean of each replicate, with each replicate being an average of a similar number of organoids. Recently, several publications have provided guidelines to improve reproducibility, including general biological reporting and accurate statistics221,222,223. SuperPlots is recommended for data visualization, as it shows both the mean of each independent biological replicate and the spread of the individual data points224,225. This type of plot is ideal for organoid reporting, as it allows immediate visualization of any potential artefact concerning batch variation. Journal collections that deepen all aspects of statistics used in biological sciences are valuable resources to facilitate accurate reporting226.
为了提高结果的重现性和可靠性,必须考虑不同细胞分离之间的变异性和类器官内异质性。尽管人们可以认为至少三个具有至少三个不同生物重复的独立实验是最低要求,但这可能并不总是足够的。事实上,每个生物重复要研究的类器官数量以及生物重复的数量取决于研究问题、具体实验和观察到的表型的变异性。只要有可能,强烈建议计算每个生物重复的独立类器官数量,考虑实验的统计功效和方差,进行类似于小鼠实验的样本量计算。数据应该是稳健的,这意味着在至少三个独立的生物重复中重复,这是指从不同动物或患者中独立分离。然而,每个实验的重复和研究问题应提前确定,以避免假设钓鱼和增加重复以获得条件 219,220 之间的显着性。数据应正确报告为每次重复的平均值,每个重复都是相似数量的类器官的平均值。最近,一些出版物提供了提高可重复性的指南,包括一般生物学报告和准确统计数据 221,222,223。建议将 SuperPlots 用于数据可视化,因为它显示了每个独立生物重复的平均值和单个数据点 224,225 的分布。 这种类型的图是类器官报告的理想选择,因为它允许立即可视化与批次变异有关的任何潜在伪影。深化生物科学统计学各个方面的期刊收藏是促进准确报告的宝贵资源 226。
Organoids should be characterized in full, not only checked for the expression of markers but also benchmarked to the tissue of origin. It is crucial to not only determine whether cells have similar morphology and expression patterns as the tissue of origin and that they have a similar function (using, for example, ELISA for albumin, bile acid production by liver/hepatocyte organoids). Whenever possible, results between compared conditions should be normalized against DNA content (for genomic analysis) or structural proteins (such as actin, for western blotting), whereas functional data such as from ELISA should be normalized to the total number of cells or area of organoid structures in each well being compared. Improving reporting standards is the first step towards increasing reproducibility, thus enabling the field to move forward.
类器官应进行全面表征,不仅检查标记物的表达,而且还应以来源组织为基准。至关重要的是,不仅要确定细胞是否具有与起源组织相似的形态和表达模式,并且它们是否具有相似的功能(例如,使用白蛋白、肝脏/肝细胞类器官产生胆汁酸的 ELISA)。只要有可能,比较条件之间的结果应根据 DNA 含量(用于基因组分析)或结构蛋白(例如肌动蛋白,用于蛋白质印迹)进行归一化,而来自 ELISA 的功能数据应归一化为细胞总数或每个被比较孔中类器官结构的面积。提高报告标准是提高可重复性的第一步,从而使该领域能够向前发展。
Data deposition 数据沉积
Standardized organoid data repositories are currently missing. A notable exception is a patient-derived repository for cancer organoids led by the National Cancer Institute (NCI) in the USA and the Human Cancer Models Initiative (HCMI), a combined international effort of several institutions (Cancer Research UK; NCI; Hubrecht Organoid Technology’s the HUB; and the US Office of Cancer Genomics’ OCG) (Supplementary Table 3). However, healthy patient-derived organoids and organoid models from non-cancer diseases, both human and murine, are yet to be documented in one database, indicating the apparent need to fully document, report and deposit all organoid lines generated. The recent launch of the Human Cell Atlas-Organoid initiative, documenting all human-derived organoids227, is the first step in that direction. For transcriptomics-related data, such as gene expression, non-coding RNA, ChIP, genome methylation, high-throughput PCR with reverse transcription, SNP arrays, SAGE and protein arrays, the Gene Expression Omnibus (GEO) is generally recommended228,229. For code used to analyse organoid data sets, GitHub is recommended. We envision that data and organoid depositories will arise shortly to fill in the presently missing gap in data repositories for organoid work. When depositing data from human organoids, it is important to consider all ethical regulations and the necessity of obtaining patient consent and keeping patient anonymity. Critical points have been extensively reviewed elsewhere230,231.
目前缺少标准化的类器官数据存储库。一个值得注意的例外是由美国国家癌症研究所 (NCI) 和人类癌症模型倡议 (HCMI) 领导的癌症类器官患者来源库,这是多个机构(英国癌症研究中心;NCI;Hubrecht Organoid Technology 的 HUB;和美国癌症基因组学办公室的 OCG)(补充表 3)。然而,来自非癌症疾病(包括人类和小鼠)的健康患者来源的类器官和类器官模型尚未记录在一个数据库中,这表明显然需要完整记录、报告和存放所有产生的类器官系。最近启动的人类细胞图谱类器官计划记录了所有人类来源的类器官 227,是朝着这个方向迈出的第一步。对于转录组学相关数据,例如基因表达、非编码 RNA、ChIP、基因组甲基化、逆转录高通量 PCR、SNP 阵列、SAGE 和蛋白质阵列,通常推荐基因表达综合(GEO)228,229。对于用于分析类器官数据集的代码,建议使用 GitHub 。我们设想,数据和类器官储存库将很快出现,以填补目前类器官工作数据存储库中缺失的空白。在从人类类器官存入数据时,重要的是要考虑所有伦理法规以及获得患者同意和保持患者匿名的必要性。临界点已在其他地方进行了广泛审查 230,231。
A consensus in organoid nomenclature is also missing. Aside from a few notable examples, where leaders in the intestine, liver and pancreas addressed the nomenclature of gut, hepatic, pancreatic and biliary organoids232,233, the field is waiting for a more clearly defined nomenclature system.
类器官命名法也缺乏共识。除了一些值得注意的例子,其中肠道、肝脏和胰腺的领导者解决了肠道、肝脏、胰腺和胆道类器官的命名法 232,233,该领域正在等待一个更明确定义的命名系统。
Limitations and optimizations
限制和优化
The reproducibility, both morphological and functional, of the obtained 3D organoid systems remains a major bottleneck. In this section, we elaborate on the limitations and recent developments in organoid research, providing a path towards a more optimal pipeline for developing, characterizing and benchmarking organoid systems.
所获得的 3D 类器官系统的形态学和功能可重复性仍然是一个主要瓶颈。在本节中,我们详细阐述了类器官研究的局限性和最新发展,为开发、表征和基准测试类器官系统提供了一条更优化的管道。
Limited level of maturity and function
成熟度和功能水平有限
None of the present organoid model systems reproduces the entire physiological repertoire of cell types, maturation level and/or function of their respective organ; they, rather, exhibit certain functions of the tissue they predominantly form. The vast majority of tissue-derived organoid models are missing tissue-specific cell types, including niche-specific mesenchyme, immune cells, vascularization, innervation or microbiome. Recently, ductal cell–liver mesenchymal cell co-cultures have been shown to recapitulate part of the liver portal tract architecture73. Specifically challenging is that not all cell types have the same proliferation rate, growth factor requirements or even requirements for oxygen exposure (hypoxia for vasculature). Pluripotent stem cell-derived organoids are better in recapitulating different cell types and cellular interactions of the developing organ but fail on exhibiting adult-tissue structures and functions, as well as cell maturation. One strategy that helps is in vivo transplantation234. However, this is at the expense of giving up control over the formed tissue constructs. Meanwhile, optimizations on differentiation protocols are to enrich maturation and specific functions of interest.
目前的类器官模型系统都没有再现其各自器官的细胞类型、成熟水平和/或功能的全部生理库;相反,它们表现出它们主要形成的组织的某些功能。绝大多数组织来源的类器官模型都缺少组织特异性细胞类型,包括生态位特异性间充质、免疫细胞、血管化、神经支配或微生物组。最近,导管细胞-肝间充质细胞共培养已被证明可以概括肝门静脉结构的一部分 73。特别具有挑战性的是,并非所有细胞类型都具有相同的增殖率、生长因子需求,甚至对氧气暴露的要求(脉管系统的缺氧)。多能干细胞衍生的类器官在概括发育器官的不同细胞类型和细胞相互作用方面表现得更好,但未能表现出成体组织结构和功能以及细胞成熟。一种有帮助的策略是体内移植 234。然而,这是以放弃对已形成的组织结构的控制为代价的。同时,对分化协议的优化是为了丰富成熟度和感兴趣的特定功能。
Another factor contributing to limited maturity and function is the nutrient (in)accessibility and accumulation of dead cells in hollow lumens. This is particularly important for iPSC-derived organoids. As organoids grow in size, the nutrient supply to cells localized in the centre of the organoid gets restricted, resulting in cellular death. This is common with organoids that form a more compact structure, such as brain organoids. For tissue-derived organoids forming a hollow cyst (cholangiocyte, pancreas), dead cells will eventually start accumulating in their lumens, which cannot be avoided but can be resolved by mechanical fragmentation of organoids. Constant fragmentation of formed structures prevents carrying out the long-term studies. However, pluripotent stem cell-derived organoids cannot be fragmented and passaged; and new strategies to solve the nutrient accessibility problem are being developed, including the long-term maintenance of brain slices in vitro235.
导致成熟度和功能受限的另一个因素是中空腔中死细胞的营养(不)可及性和积累。这对于 iPSC 衍生的类器官尤为重要。随着类器官体积的增长,位于类器官中心的细胞的营养供应受到限制,导致细胞死亡。这在形成更紧凑结构的类器官中很常见,例如脑类器官。对于形成中空囊肿的组织来源的类器官(胆管细胞、胰腺),死细胞最终会开始在其管腔中积聚,这是无法避免的,但可以通过类器官的机械碎片化来解决。形成结构的不断破碎阻碍了进行长期研究。然而,多能干细胞衍生的类器官不能被片段化和传代;并且正在开发解决营养可及性问题的新策略,包括体外脑切片的长期维护 235。
Limited control of heterogeneity
对异质性的有限控制
Once cells form an organoid, we have minimal input in cellular behaviour within the organoid. Even in the same experimental settings, the result is often a plethora of phenotypic traits (shape, size, cell composition) rather than a stereotypic culture. Optimizing morphogenic gradients, tissue-specific cell–ECM interaction and local biochemical and biophysical properties are essential for minimizing batch to batch heterogeneity236.
一旦细胞形成类器官,我们对类器官内细胞行为的输入就很少。即使在相同的实验环境中,结果通常也是过多的表型特征(形状、大小、细胞组成),而不是刻板培养。优化形态发生梯度、组织特异性细胞-ECM 相互作用以及局部生化和生物物理特性对于最大限度地减少批次间异质性至关重要 236。
To generate more complex multicellular mature and functional structures, the organoid field has started to create assembloids as demonstrated for human cortico-motor assembloids237. Such effort allows the creation of more complex structures, connecting multiple types of tissues with a defined interface such as connecting cerebral cortex, spine and skeletal muscle with neuro-muscular junctions, but at the cost of reproducibility. As recently discussed in another review focused on hepatic, biliary and pancreatic organoids233, reproducibility in multicellular and multi-tissue organoid systems decreases as it is challenging to coordinate proliferation and differentiation of multiple cell types.
为了产生更复杂的多细胞成熟和功能结构,类器官领域已经开始产生组合体,如人类皮质运动组合体 237 所证明的那样。这种努力允许创建更复杂的结构,将多种类型的组织与定义的界面连接起来,例如将大脑皮层、脊柱和骨骼肌与神经肌肉接头连接起来,但以牺牲可重复性为代价。正如最近在另一篇专注于肝脏、胆道和胰腺类器官的综述中所讨论的那样 233,多细胞和多组织类器官系统的可重复性降低,因为协调多种细胞类型的增殖和分化具有挑战性。
The limited control of intra-organoid heterogeneity is detrimental for high-throughput screening applications and makes it difficult for studies requiring high spatio-temporal resolution imaging. Instead of creating more complex organoid systems, simpler models of reduced dimensions to recapitulate the essential tissue structures and functions of interest are gaining momentum. Variants of ECM combinations, micropatterned 2D mono-culture or co-culture238,239, cell sheets240, stacked 3D structures25 and micro-positioned ECM substrates69,241 allow the formation of reproducible tissue structures and functions with a high degree of spatio-temporal control such as stretching242 and osmotic forces243 (Fig. 5).
类器官内异质性的有限控制不利于高通量筛选应用,并使需要高时空分辨率成像的研究变得困难。没有创建更复杂的类器官系统,而是缩小尺寸的更简单的模型来概括感兴趣的基本组织结构和功能,正在获得动力。ECM 组合的变体,微图案化的 2D 单培养或共培养 238,239,细胞片 240,堆叠的 3D 结构 25 和微定位的 ECM 底物 69,241 允许形成可重复的组织结构和功能,并具有高度的时空控制,例如拉伸 242 和渗透力 243(图 5 )。
图5:通过降低复杂性来减少异质性。
a–c, The organoid development process includes the formation of an initial 2D condition (part a), growth into a 3D structure (part b) and tissue morphogenesis (part c). d,e, Simpler models of reduced dimensions to recapitulate the essential tissue structures and functions of interest are gaining momentum. Micropatterned 2D mono-culture or co-culture allows the formation of a reproducible initial 2D condition which can further form the initial 3D structure. For example, crypt formation can be controlled by localized matrix softening and geometry can induce patterned differentiation with enterocytes (L-FABP stain) in the centre region and proliferating LGR5 cells in the buds69 (part d). Subsequently, a high degree of spatio-temporal control can be applied to direct certain tissue morphogenesis. For example, neural cell differentiation pattern can be modulated by stretch magnitude and timing242. Graph represents the stretch magnitude form 0 to 100% and scattered (S) and patterned (P) are the two configurations of differentiation patterning. f, Osmotic forces can control the crypt morphology in intestinal organoids243. Scale bars: 30 μm (part d), 50 μm (parts e and f ). Blebb, blebbistatin; hNTO, human neural tube organoid; PGE, prostaglandin E2. Part d adapted with permission from ref.69, AAAS. Part e adapted from ref.242, CC BY 4.0. Part f adapted from ref.243, Springer Nature Limited.
a-c,类器官发育过程包括初始 2D 条件的形成(a 部分)、生长成 3D 结构(b 部分)和组织形态发生(c 部分)。d,e,用于概括感兴趣的基本组织结构和功能的更简单的缩小尺寸模型正在获得动力。微图案化的 2D 单培养或共培养允许形成可重复的初始 2D 条件,从而进一步形成初始 3D 结构。例如,隐窝的形成可以通过局部基质软化来控制,几何形状可以诱导中心区域的肠细胞(L-FABP 染色)和芽 69(d 部分)中增殖的 LGR5 细胞的模式化分化。随后,可以应用高度的时空控制来指导某些组织形态发生。例如,神经细胞分化模式可以通过拉伸幅度和时间 242 进行调节。图表示拉伸幅度,从 0 到 100%,散射(S)和图案化(P)是微分图案的两种配置。f,渗透力可以控制肠道类器官中的隐窝形态 243。比例尺:30 μm(d 部分)、 50 μm(e 和 f 部分)。Blebb,blebbistatin;hNTO, 人神经管类器官; PGE,前列腺素 E2。d 部分经 ref.69, AAAS 许可改编。e 部分改编自 ref.242,CC BY 4.0。f 部分改编自 Springer Nature Limited 的 ref.243。
Optimizing ECM composition
优化 ECM 组成
Engineering methods have been implemented to optimize these limitations (Fig. 6). There are two main paths to overcome the use of non-specific ECM such as Matrigel: one is the use of synthetic matrices with more complete control over composition and stiffness, and the other is to take decellularized tissue and create tissue-specific matrices207,244. There are significant efforts to identify chemically defined, GMP-compatible ECMs that enable the growth and the long-term expansion of human organoids. In that regard, some advances were shown with human pancreas organoids, intestinal organoids and colorectal cancer organoids, which could grow in a dextran-based fully defined ECM; however, they would not expand long term3,68.
已经实施了工程方法来优化这些限制(图。6).克服使用非特异性 ECM(如基质胶)有两种主要途径:一种是使用对成分和刚度有更完全控制的合成基质,另一种是取脱细胞组织并创建组织特异性基质 207,244。人们正在做出重大努力来确定化学定义的、符合 GMP 的 ECM,这些 ECM 能够实现人类类器官的生长和长期扩增。在这方面,人胰腺类器官、肠道类器官和结直肠癌类器官显示出一些进展,它们可以在基于葡聚糖的完全定义的 ECM 中生长;然而,他们不会长期扩张 3,68。
图6:类器官培养的当前局限性以及克服这些局限性的方法的并排比较。
a, Accumulation of dead cells and cell debris inside cystic organoid lumina (left) has been overcome by designing perfusable open-end structures (right) that use inducible flow to wash out cell debris, which are more compatible with long-term experiments. b, Organoids grown in Matrigel domes display high variability of cell heterogeneity and morphology (left), which can be overcome by using grids with patterned synthetic extracellular matrix (ECM) (right) that provide cues for cell differentiation. These platforms are additionally compatible with high-throughput screenings. c, Single cell type-derived organoids do not recapitulate the cellular and physiological complexity of native tissue (left), but combining organoids with organ-on-a-chip (OoC) (right) as a novel technology would enable creating a controlled microenvironment, suitable for multiple cell types.
a,通过设计可灌注的开放端结构(右),使用诱导流冲洗掉细胞碎片,克服了囊性类器官管腔内死细胞和细胞碎片的积累(左),这更适合长期实验。b,在基质胶圆顶中生长的类器官表现出细胞异质性和形态的高度变异性(左),这可以通过使用具有图案化合成细胞外基质(ECM)(右)的网格来克服,这些网格为细胞分化提供线索。这些平台还与高通量筛选兼容。c,单细胞类型衍生的类器官不能概括天然组织的细胞和生理复杂性(左),但将类器官与器官芯片(OoC)(右)作为一项 新技术相结合,将能够创建一个适用于多种细胞类型的受控微环境。
Organoids meet organs-on-a-chip
类器官与芯片上的器官相遇
It has been shown that by maximizing the mass transfer and minimizing the shear stress in the perfusive soluble microenvironment, cells grown in OoC set-ups upregulate their functions, getting a step closer to a native tissue93,245,246. A more recent example shows how the presence of fluid flow enhances kidney organoids’ maturation and favours their vascularization in vitro106. Physical constraints were engineered into the organoid environment and intestinal cells when provided with boundaries through engineered scaffolds self-organized in crypts of the same size. At the same time, they overcame the inaccessibility of cystic organoids and clearance of cell debris by creating a perfusable culture of mini-intestines where cells are arranged to form tube-shaped epithelia and similar spatial arrangement to the in vivo tissue99.
已经表明,通过最大化传质和最小化灌注可溶性微环境中的剪切应力,在 OoC 设置中生长的细胞上调其功能,更接近天然组织 93,245,246。最近的一个例子表明,液体流动的存在如何促进肾脏类器官的成熟并有利于其在体外的血管化 106。当通过在相同大小的隐窝中自组织的工程支架提供边界时,物理约束被设计到类器官环境和肠道细胞中。同时,他们通过创建可灌注的小肠培养物来克服囊性类器官的不可接近性和细胞碎片的清除,其中细胞排列形成管状上皮细胞,并具有与体内组织相似的空间排列 99。
Outlook 展望
Moving forward, the trend is to develop more complex models that recapitulate in vivo structure and function as faithfully as possible, in terms of the recapitulating cell types over time, tissue architecture, measurable molecular events and phenotypic functions. Rather than focusing exclusively on the most prominent markers or functional assays, architectural benchmarking of native tissue should also be performed. In the example of hepatocyte organoids124, hepatocyte functions are preserved, yet the liver tissue architecture does not match the native tissue where hepatocytes are arranged in cords. Similarly, organoids such as pancreatic or colon cancer organoids grow isotropically, forming a cyst instead of the tubular structure they would form in their native tissue. To derive more complex functions, organoids with multicellular and multi-tissue structures will be important, especially in the context of studying cell–cell interactions247. Along this vein, assembloids and organs-on-chips are also becoming increasingly complex and more broadly adopted.
展望未来,趋势是开发更复杂的模型,在随时间推移的细胞类型、组织结构、可测量的分子事件和表型功能方面尽可能忠实地概括体内结构和功能。与其只关注最突出的标记物或功能测定,还应对天然组织进行结构基准测试。在肝细胞类器官 124 的例子中,肝细胞功能得以保留,但肝组织结构与肝细胞排列在脐带中的天然组织不匹配。类似地,胰腺癌或结肠癌类器官等类器官各向同性生长,形成囊肿,而不是它们在天然组织中形成的管状结构。为了获得更复杂的功能,具有多细胞和多组织结构的类器官将很重要,特别是在研究细胞间相互作用的背景下 247。沿着这条脉络,组件和器官芯片也变得越来越复杂,并且被更广泛地采用。
On the other hand, the engineer’s approach27,248 is to pursue simpler reductionist models defined by the minimal functional modules that drive a complex cellular or tissue function of interest, to study the mechano-biological causation in development or repair, or to develop a robust system for high-throughput screening. The basic premise is that a complex biological function is executed by coordinated operation of a limited number of functional modules, each described by a small set of molecules, and chemical reactions driving physical attribute changes of mesoscale (subcellular or intercellular tissue/multicellular) structures associated with functions of interest in the specific spatio-temporal stage/phase/step. For example, bile canaliculi in the liver exhibit hourly cycles of expansion and contraction. To study the causative contraction events with high resolution, only regions of adjacent hepatocytes forming the bile canaliculi are directly studied in the context of the entire regulatory machinery of adjacent hepatocytes26,249. One can choose to create a much larger structure involving cholangiocytes than the one operated by the minimal functional modules but the model will be noisy and costly. Each functional module is coupled to another and can be modelled together or independently at different length scale. Simple reductionist models have been useful for high-resolution mechanistic understanding of tissue morphogenetic events such as defects28,241,250.
另一方面,工程师的方法 27,248 是追求由驱动感兴趣的复杂细胞或组织功能的最小功能模块定义的更简单的还原论模型,研究发育或修复中的机械生物学因果关系,或开发用于高通量筛选的稳健系统。基本前提是,复杂的生物功能是通过有限数量的功能模块的协调作来执行的,每个功能模块由一小组分子描述,以及驱动与特定时空阶段/阶段/步骤中感兴趣的功能相关的介尺度(亚细胞或细胞间组织/多细胞)结构的物理属性变化的化学反应。例如,肝脏中的胆管表现出每小时一次的扩张和收缩周期。为了以高分辨率研究致病收缩事件,仅在相邻肝细胞的整个调节机制的背景下直接研究形成胆管的相邻肝细胞区域 26,249。人们可以选择创建一个比由最小功能模块作的结构更大的涉及胆管细胞的结构,但该模型会嘈杂且成本高昂。每个功能模块都与另一个功能模块耦合,可以一起建模,也可以以不同的长度尺度独立建模。简单的还原模型对于组织形态发生事件(例如缺陷 28,241,250)的高分辨率机制理解非常有用。
Geometrically constraining the size of the initial 2D seeding pattern and 3D formation by micropatterning and supporting 3D cell growth using Matrigel enabled the induction of tissue-like neural tube morphogenesis and production of highly reproducible neural tubes. This also allowed identification of the mechanisms of neural tube folding and modelled neural tube defects25. In another example, symmetry breaking in a uniform sphere of cells and the emergence of a Paneth cell is a critical event in the early stage of intestinal organoid formation. The mechanism was recently elucidated: symmetry breaking is caused by transient activation of the mechanotransducer YAP1, which induces NOTCH-DLL1 lateral inhibition events206. YAP1 activation has since been controlled by applying geometrical constraints in hydrogel scaffolds and producing a uniform and reproducible intestinal microtissue3.
通过微图案化和使用基质胶支持 3D 细胞生长,对初始 2D 接种模式和 3D 形成进行几何限制,从而能够诱导组织样神经管形态发生并产生高度可重复的神经管。这也允许识别神经管折叠的机制和建模的神经管缺陷 25。在另一个例子中,均匀细胞球体中的对称性被打破和潘氏细胞的出现是肠道类器官形成早期阶段的关键事件。该机制最近被阐明:对称性破缺是由机械换能器 YAP1 的瞬时激活引起的,该激活诱导 NOTCH-DLL1 横向抑制事件 206。此后,YAP1 激活通过在水凝胶支架中应用几何约束并产生均匀且可重复的肠道微组织 3 来控制。
Organoids can be constrained by reducing the third dimension in a 2.5D culture. The 2.5D culture reduces the depth-driven variabilities of a typical organoid: diffusional constraints in the hypoxic core, limited accessibility for drugs/transfection agents and impeded imaging transparency251. Typical examples of restrictions in the third dimension include culturing cells on curved or patterned surfaces, a flattened or constrained cellular construct251 and overlaying the ECM on a flat cell monolayer at high confluency, which would pull the cells upward, forcing more cell–cell interactions to adopt 3D cell morphology. Hepatocytes in a collagen sandwich have sufficient contact area to attain polarity and form a bile canalicular lumen that contracts in the same periodic cycles as in vivo without the 3D network, wider and cholestatic compared with the native tissue. This cell-based model enables high-resolution dissection of the mechanism of bile canaliculi contraction into steps and an understanding of the molecular machinery regulating the phase transitions26,252. We would also envision more engineered organoid models based on CRISPR-edited cells for disease modelling, although these cells and models are synthetic.
类器官可以通过减少 2.5D 培养物中的三维来限制。2.5D 培养减少了典型类器官的深度驱动变异性:缺氧核心中的扩散限制,药物/转染剂的可及性有限以及成像透明度受阻 251。三维限制的典型例子包括在弯曲或图案化表面上培养细胞、扁平或受约束的细胞构建体 251 以及以高汇合度将 ECM 覆盖在扁平细胞单层上,这将将细胞向上拉,迫使更多的细胞间相互作用采用 3D 细胞形态。胶原蛋白夹心中的肝细胞具有足够的接触面积来达到极性并形成胆管腔,该胆管腔在与没有 3D 网络的体内相同的周期周期内收缩,与天然组织相比更宽且 胆汁淤积。这种基于细胞的模型能够将胆管收缩机制高分辨率剖析为步骤,并了解调节相变的分子机制 26,252。我们还设想更多基于 CRISPR 编辑细胞的工程类器官模型用于疾病建模,尽管这些细胞和模型是合成的。
Besides technological advances in creating more physiologically relevant, robust and easier to use organoid models, we envision seeing greater impact in applications. In the past two decades, although there have been discussions to replace animal testing, these efforts have not yet led to concrete actions. However, this is rapidly changing, with regulatory hard stops now established on multiple fronts253. Organoids that can recapitulate the complex physiological functions in vivo have also boosted confidence that the new alternative methods are now viable options. There will be more extrapolation of animal research findings in human organoids to better understand human biology and pathophysiology. We envision widespread adoption of organoids as cell sources for cell therapy, regenerative medicine, in vitro diagnostics and drug discovery.
除了在创建更具生理相关性、更稳健和更易于使用的类器官模型方面的技术进步外,我们还预计在应用中会产生更大的影响。在过去的二十年里,尽管有人讨论过取代动物试验,但这些努力尚未导致具体行动。然而,这种情况正在迅速改变,现在在多个方面建立了监管硬止损 253。可以在体内概括复杂生理功能的类器官也增强了人们对新的替代方法现在是可行选择的信心。将对人类类器官的动物研究结果进行更多推断,以更好地了解人类生物学和病理生理学。我们设想类器官将广泛采用作为细胞治疗、再生医学、体外诊断和药物发现的细胞来源。
References 引用
Zakrzewski, W., Dobrzynski, M., Szymonowicz, M. & Rybak, Z. Stem cells: past, present, and future. Stem Cell Res. Ther. 10, 68 (2019).
Zakrzewski, W.、Dobrzynski, M.、Szymonowicz, M. 和 Rybak, Z. 干细胞:过去、现在和未来。 干细胞研究。10, 68 (2019)。Voog, J. & Jones, D. L. Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115 (2010).
Voog, J. & Jones, DL 干细胞和生态位:充满活力的二人组。 细胞干细胞 6, 103–115 (2010)。Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016). This study introduces the concept of the stem cell niche, and describes that there are different requirements for mechanical cues at different stages of intestinal organoid formation.
Gjorevski, N. 等人。用于肠道干细胞和类器官培养的设计基质。 自然 539, 560–564 (2016)。 本研究介绍了干细胞生态位的概念,并描述了在肠道类器官形成的不同阶段对机械线索有不同的要求。Yi, S. A., Zhang, Y., Rathnam, C., Pongkulapa, T. & Lee, K. B. Bioengineering approaches for the advanced organoid research. Adv. Mater. 33, e2007949 (2021).
Yi, SA, Zhang, Y., Rathnam, C., Pongkulapa, T. & Lee, KB 高级类器官研究的生物工程方法。 副主教。33, e2007949 (2021)。Orkin, R. et al. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 145, 204–220 (1977).
Orkin, R. 等人。产生基底膜基质的小鼠肿瘤。J. Exp. 医学。145, 204–220 (1977)。Li, M. L. et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. Natl Acad. Sci. USA 84, 136–140 (1987). This work demonstrates that Matrigel could support in vitro 3D culture and cell functions.
李,ML 等人。重构基底膜及其组分对小鼠乳腺上皮细胞酪蛋白基因表达和分泌的影响。 美国国家科学院 84, 136–140 (1987)。 这项工作表明,基质胶可以支持体外 3D 培养和细胞功能。Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). This work shows the stem cells’ potential to recapitulate native tissue-like structures and functions.
Sato, T. et al. 单个 Lgr5 干细胞在体外构建隐窝-绒毛结构,没有间充质生态位。 自然 459, 262–265 (2009)。 这项工作显示了干细胞概括天然组织样结构和功能的潜力。Huch, M. & Koo, B.-K. Modeling mouse and human development using organoid cultures. Development 142, 3113–3125 (2015).
Huch, M. 和 Koo, B.-K.使用类器官培养物模拟小鼠和人类发育。 发展 142, 3113–3125 (2015)。Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Lancaster, MA 和 Knoblich, JA 从人类多能干细胞生成脑类器官。 国家协议。9, 2329–2340 (2014)。Simian, M. & Bissell, M. J. Organoids: a historical perspective of thinking in three dimensions. J. Cell Biol. 216, 31–40 (2017).
Simian, M. 和 Bissell, MJ 类器官:三维思维的历史视角。J. 细胞生物学。216, 31–40 (2017)。Reis, R. L. 2nd Consensus conference on definitions on biomaterials science. J. Tissue Eng. Regen. Med. 14, 561–562 (2020).
Reis, RL 生物材料科学定义的第二次共识会议。J. Tissue Eng. Regen. Med.14, 561–562 (2020)。Hofer, M. & Lutolf, M. P. Engineering organoids. Nat. Rev. Mater 6, 402–420 (2021). This review describes current limitations of organoid culture and proposed engineering approaches to address these limitations.
Hofer, M. & Lutolf, M. P. 工程类器官。Nat. Rev. Mater6, 402–420 (2021)。 本综述描述了类器官培养的当前局限性以及解决这些局限性的工程方法。Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Clevers, H. 用类器官模拟发育和疾病。 细胞 165, 1586–1597 (2016)。Kim, M. et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 10, 3991 (2019). This work establishes personalized lung cancer organoids and normal bronchial organoids from patient tissues.
Kim, M. 等人。患者来源的肺癌类器官作为用于治疗筛查的体外癌症模型。 国家公社。10, 3991 (2019)。 这项工作从患者组织中建立了个性化的肺癌类器官和正常支气管类器官。Al Shihabi, A. et al. Personalized chordoma organoids for drug discovery studies. Sci. Adv. 8, eabl3674 (2022). This publication is the first showing that patient-derived tumour organoids can be established for rare, indolent cancers and maintain the original tissue’s morphological and functional profiles.
Al Shihabi, A. 等人。用于药物发现研究的个性化脊索瘤类器官。 科学。8、EABL3674 (2022)。 该出版物首次表明可以为罕见的惰性癌症建立患者来源的肿瘤类器官,并维持原始组织的形态和功能特征。Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D. & Brivanlou, A. H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847–854 (2014).
Warmflash, A.、Sorre, B.、Etoc, F.、Siggia, E. D. 和 Brivanlou, A. H.一种概括人类胚胎干细胞早期胚胎空间模式的方法。 国家方法 11, 847–854 (2014)。Wang, X., Liu, Z. & Pang, Y. Concentration gradient generation methods based on microfluidic systems. RSC Adv. 7, 29966–29984 (2017).
Wang, X., Liu, Z. & Pang, Y. 基于微流体系统的浓度梯度生成方法。RSC Adv.7, 29966–29984 (2017)。Kumar, A., Placone, J. K. & Engler, A. J. Understanding the extracellular forces that determine cell fate and maintenance. Development 144, 4261–4270 (2017).
Kumar, A.、Placone, JK 和 Engler, AJ 了解决定细胞命运和维持的细胞外力。 发展 144, 4261–4270 (2017)。White, C. R. & Frangos, J. A. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 1459–1467 (2007).
怀特,CR 和弗兰戈斯,JA 这一切的剪切应力:细胞膜和机械化学转导。Philos. Trans. R. Soc. Lond.B 生物学。362, 1459–1467 (2007)。Vatine, G. D. et al. Human iPSC-derived blood–brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24, 995–1005.e6 (2019).
瓦廷,GD 等人。人 iPSC 衍生的血脑屏障芯片支持疾病建模和个性化医疗应用。 细胞干细胞 24, 995–1005.e6 (2019)。Teng, Y., Zhao, Z., Tasnim, F., Huang, X. & Yu, H. A scalable and sensitive steatosis chip with long-term perfusion of in situ differentiated HepaRG organoids. Biomaterials 275, 120904 (2021).
Teng, Y., Zhao, Z., Tasnim, F., Huang, X. &; Yu, H.一种可扩展且灵敏的脂肪变性芯片,可长期灌注原位分化的 HepaRG 类器官。 生物材料 275, 120904 (2021)。Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
钱,X.等人。使用微型生物反应器模拟寨卡病毒暴露的脑区特异性类器官。 细胞 165, 1238–1254 (2016)。Ladoux, B. & Mege, R. M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 18, 743–757 (2017).
Metavarayuth, K., Sitasuwan, P., Zhao, X., Lin, Y. & Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 2, 142–151 (2016).
Karzbrun, E. et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268–272 (2021). This work presents a chip-based culture system that enables self-organization of micropatterned stem cells into precise 3D cell fate patterns and organ shapes.
Gupta, K. et al. Bile canaliculi contract autonomously by releasing calcium into hepatocytes via mechanosensitive calcium channel. Biomaterials 259, 120283 (2020).
Sheetz, M. & Yu, H. The Cell as a Machine (Cambridge Univ. Press, 2018). This book explains the rationale of mechanobiology and the approaches to dissect complex biological functions.
Saw, T. B. et al. Topological defects in epithelia govern cell death and extrusion. Nature 544, 212–216 (2017).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
Bartfeld, S. et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136.e6 (2015).
Aihara, E. et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci. Rep. 5, 1–15 (2015).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013). This study describes the first liver organoid cultures from adult mouse liver tissue, where cells can be expanded long term to form liver organoids even from a single cell.
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Greggio, C. et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140, 4452–4462 (2013). This work presents the first fetal tissue organoid culture system that recapitulates pancreas progenitor development.
Huch, M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R–spondin axis. EMBO J. 32, 2708–2721 (2013). This study is the first to produce pancreas organoids from adult tissue.
Manzar, G. S., Kim, E. M. & Zavazava, N. Demethylation of induced pluripotent stem cells from type 1 diabetic patients enhances differentiation into functional pancreatic β cells. J. Biol. Chem. 292, 14066–14079 (2017).
Drakhlis, L. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 39, 737–746 (2021).
Koehler, K. R., Mikosz, A. M., Molosh, A. I., Patel, D. & Hashino, E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013). This work presents the first inner ear organoids developed from iPSCs.
Lee, J. et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399–404 (2020).
Beers, J. et al. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 7, 2029–2040 (2012).
Hu, P., Zhang, W., Xin, H. & Deng, G. Single cell isolation and analysis. Front. Cell Dev. Biol. 4, 116 (2016).
Aronowitz, J. A., Lockhart, R. A. & Hakakian, C. S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 4, 713 (2015).
Gaipl, U. S. et al. Cooperation between C1q and DNase I in the clearance of necrotic cell-derived chromatin. Arthritis Rheum. 50, 640–649 (2004).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Kopper, O. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 25, 838–849 (2019).
Tiriac, H. et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest. Endosc. 87, 1474–1480 (2018).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Seino, T. et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22, 454–467.e6 (2018).
Mazzocchi, A. et al. Pleural effusion aspirate for use in 3D lung cancer modeling and chemotherapy screening. ACS Biomater. Sci. Eng. 5, 1937–1943 (2019).
Lohmussaar, K. et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 28, 1380–1396.e6 (2021).
Sartini, S. & Soragni, A. Cervical organoids go viral. Cell Stem Cell 28, 1337–1338 (2021).
De Angelis, M. L. et al. An organoid model of colorectal circulating tumor cells with stem cell features, hybrid EMT state and distinctive therapy response profile. J. Exp. Clin. Cancer Res. 41, 86 (2022).
Bergin, C. J. & Benoit, Y. D. Protocol for serial organoid formation assay using primary colorectal cancer tissues to evaluate cancer stem cell activity. STAR. Protoc. 3, 101218 (2022).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Nguyen, H. T. L. & Soragni, A. Patient-derived tumor organoid rings for histologic characterization and high-throughput screening. STAR. Protoc. 1, 100056 (2020).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Peng, H., Poovaiah, N., Forrester, M., Cochran, E. & Wang, Q. Ex vivo culture of primary intestinal stem cells in collagen gels and foams. ACS Biomater. Sci. Eng. 1, 37–42 (2015).
Cruz-Acuña, R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017).
Kratochvil, M. J. et al. Engineered materials for organoid systems. Nat. Rev. Mater. 4, 606–622 (2019).
Broguiere, N. et al. Growth of epithelial organoids in a defined hydrogel. Adv. Mater. 30, 1801621 (2018).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
Chrisnandy, A., Blondel, D., Rezakhani, S., Broguiere, N. & Lutolf, M. P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 21, 479–487 (2021).
Georgakopoulos, N. et al. Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev. Biol. 20, 4 (2020).
Gjorevski, N. et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021 (2022).
Valamehr, B. et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc. Natl Acad. Sci. USA 105, 14459–14464 (2008).
Brandenberg, N. et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 4, 863–874 (2020).
Czerniecki, S. M. et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22, 929–940.e4 (2018).
Cordero-Espinoza, L. et al. Dynamic cell contacts between periportal mesenchyme and ductal epithelium act as a rheostat for liver cell proliferation. Cell Stem Cell 28, 1907–1921.e8 (2021).
Jiang, S. et al. An automated organoid platform with inter-organoid homogeneity and inter-patient heterogeneity. Cell Rep. Med. 1, 100161 (2020).
Bues, J. et al. Deterministic scRNA-seq captures variation in intestinal crypt and organoid composition. Nat. Methods 19, 323–330 (2022).
Wang, Y. et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 18, 3606–3616 (2018).
Goonoo, N. & Bhaw-Luximon, A. Mimicking growth factors: role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Adv. 9, 18124–18146 (2019).
Siller, R., Greenhough, S., Naumovska, E. & Sullivan, G. J. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 4, 939–952 (2015).
Janda, C. Y. et al. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature 545, 234–237 (2017).
Miao, Y. et al. Next-generation surrogate Wnts support organoid growth and deconvolute frizzled pleiotropy in vivo. Cell Stem Cell 27, 840–851.e6 (2020).
Ornitz, D. M. F. G. Fs heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22, 108–112 (2000).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568 (2015).
Mae, S. I. et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat. Commun. 4, 1367 (2013).
Wang, Q. et al. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv. Drug Deliv. Rev. 91, 125–140 (2015).
Peng, H., Wang, C., Xu, X., Yu, C. & Wang, Q. An intestinal Trojan horse for gene delivery. Nanoscale 7, 4354–4360 (2015).
Davoudi, Z. et al. Intestinal organoids containing poly (lactic‐co‐glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. J. Biomed. Mater. Res. A 106, 876–886 (2018).
Davoudi, Z. et al. Gut organoid as a new platform to study alginate and chitosan mediated PLGA nanoparticles for drug delivery. Mar. Drugs 19, 282 (2021).
Gjorevski, N., Ranga, A. & Lutolf, M. P. Bioengineering approaches to guide stem cell-based organogenesis. Development 141, 1794–1804 (2014).
Dalby, M. J., Gadegaard, N. & Oreffo, R. O. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 13, 558–569 (2014).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Demers, C. J. et al. Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development 143, 1884–1892 (2016).
Fluri, D. A. et al. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat. Methods 9, 509–516 (2012).
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010).
Perez-Gonzalez, C. et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 23, 745–757 (2021).
Zhang, Z.-Z. et al. Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Sci. Transl. Med. 11, eaao0750 (2019).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
Brassard, J. A., Nikolaev, M., Hübscher, T., Hofer, M. & Lutolf, M. P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 20, 22–29 (2021).
Park, S. E., Georgescu, A. & Huh, D. Organoids-on-a-chip. Science 364, 960–965 (2019).
Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020). This study shows an intestinal organoid with a similar spatial arrangement of crypt-like and villus-like domains to that in vivo.
Esch, M. B., Mahler, G. J., Stokol, T. & Shuler, M. L. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 14, 3081–3092 (2014).
Bauer, S. et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 7, 1–11 (2017).
Zhang, Y. S. et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl Acad. Sci. USA 114, E2293–E2302 (2017).
Tao, T. et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 19, 948–958 (2019).
Workman, M. J. et al. Enhanced utilization of induced pluripotent stem cell-derived human intestinal organoids using microengineered chips. Cell. Mol. Gastroenterol. Hepatol. 5, 669–677.e2 (2018).
Lee, K. K. et al. Human stomach-on-a-chip with luminal flow and peristaltic-like motility. Lab Chip 18, 3079–3085 (2018).
Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019). This study reports a microfluidic model to culture kidney organoids in vitro and shows flow-enhanced vascularization.
Grebenyuk, S. & Ranga, A. Engineering organoid vascularization. Front. Bioeng. Biotechnol. 7, 39 (2019).
Below, C. R. et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater. 21, 110–119 (2022).
Wang, D. et al. Long-term expansion of pancreatic islet organoids from resident Procr+ progenitors. Cell 180, 1198–1211 e1119 (2020). This study identifies a new stem cell population with the potential to expand into organoids for long-term culture and transplantation.
Wang, J. et al. Endothelial Wnts control mammary epithelial patterning via fibroblast signaling. Cell Rep. 34, 108897 (2021).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Friedlander, M. S. H., Nguyen, V. M., Kim, S. K. & Bevacqua, R. J. Pancreatic pseudoislets: an organoid archetype for metabolism research. Diabetes 70, 1051–1060 (2021).
Wang, J. et al. Isolation of mouse pancreatic islet Procr+ progenitors and long-term expansion of islet organoids in vitro. Nat. Protoc. https://doi.org/10.1038/s41596-022-00683-w (2022).
Ma, X. et al. Human expandable pancreatic progenitor-derived β cells ameliorate diabetes. Sci. Adv. 8, eabk1826 (2022).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Yoshihara, E. et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586, 606–611 (2020).
Date, S. & Sato, T. Mini-gut organoids: reconstitution of the stem cell niche. Annu. Rev. Cell Dev. Biol. 31, 269–289 (2015).
Rahmani, S., Breyner, N. M., Su, H. M., Verdu, E. F. & Didar, T. F. Intestinal organoids: a new paradigm for engineering intestinal epithelium in vitro. Biomaterials 194, 195–214 (2019).
Fujii, M. et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 23, 787–793.e6 (2018).
Qu, M. et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Res. 31, 259–271 (2021).
Nakamura, T. Recent progress in organoid culture to model intestinal epithelial barrier functions. Int. Immunol. 31, 13–21 (2019).
Jung, K. B. et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat. Commun. 9, 3039 (2018).
Son, Y. S. et al. Maturation of human intestinal organoids in vitro facilitates colonization by commensal Lactobacilli by reinforcing the mucus layer. FASEB J. 34, 9899–9910 (2020).
Hu, H. et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175, 1591–1606.e19 (2018). This study shows the establishment of hepatocyte organoid cultures from mouse and human fetal tissue.
Peng, W. C. et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 175, 1607–1619.e15 (2018). This study shows the establishment of hepatocyte organoid cultures for engraftment.
Lee, S. H. et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528.e17 (2018).
Yu, L. et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin. Transl. Immunol. 10, e1248 (2021).
Li, X. et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun. 9, 2983 (2018).
Honkala, A., Malhotra, S. V., Kummar, S. & Junttila, M. R. Harnessing the predictive power of preclinical models for oncology drug development. Nat. Rev. Drug Discov. 21, 99–114 (2022).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017). This study shows the first liver cancer organoid models from patient material.
Lo, Y. H., Karlsson, K. & Kuo, C. J. Applications of organoids for cancer biology and precision medicine. Nat. Cancer 1, 761–773 (2020).
Dijkstra, K. K. et al. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 31, 107588 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
McQuin, C. et al. CellProfiler 3.0: next-generation image processing for biology. PLoS Biol. 16, e2005970 (2018).
Lindeboom, R. G. et al. Integrative multi-omics analysis of intestinal organoid differentiation. Mol. Syst. Biol. 14, e8227 (2018).
Hernández-de-Diego, R. et al. PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data. Nucleic Acids Res. 46, W503–W509 (2018).
Raudvere, U. et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 47, W191–W198 (2019).
Powers, R. K., Goodspeed, A., Pielke-Lombardo, H., Tan, A. C. & Costello, J. C. GSEA-InContext: identifying novel and common patterns in expression experiments. Bioinformatics 34, i555–i564 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Reimand, J. et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 14, 482–517 (2019).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Kok, R. N. U. et al. OrganoidTracker: efficient cell tracking using machine learning and manual error correction. PLoS ONE 15, e0240802 (2020).
Mergenthaler, P. et al. Rapid 3D phenotypic analysis of neurons and organoids using data-driven cell segmentation-free machine learning. PLoS Comput. Biol. 17, e1008630 (2021).
Loomans, C. J. M. et al. Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Rep. 10, 712–724 (2018).
Elizondo, D. M. et al. Pancreatic islets seeded in a novel bioscaffold forms an organoid to rescue insulin production and reverse hyperglycemia in models of type 1 diabetes. Sci. Rep. 10, 4362 (2020).
Meran, L. et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat. Med. 26, 1593–1601 (2020).
Sugimoto, S. et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 592, 99–104 (2021).
Vives, J. & Batlle-Morera, L. The challenge of developing human 3D organoids into medicines. Stem Cell Res. Ther. 11, 72 (2020).
Phan, N. et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol. 2, 78 (2019).
Tebon, P. J. et al. Drug screening at single-organoid resolution via bioprinting and interferometry. Preprint at bioRxiv https://doi.org/10.1101/2021.10.03.462896 (2021).
Chen, J. et al. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Ther. 28, 112–125 (2021).
Verissimo, C. S. et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. eLife https://doi.org/10.7554/eLife.18489 (2016).
Nuciforo, S. et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 24, 1363–1376 (2018).
Schnalzger, T. E. et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 38, 100928 (2019).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Weeber, F. et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl Acad. Sci. USA 112, 13308–13311 (2015).
Yan, H. H. N. et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23, 882–897.e11 (2018).
Williamson, C. T. et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat. Commun. 7, 13837 (2016).
Letai, A. Functional precision cancer medicine — moving beyond pure genomics. Nat. Med. 23, 1028–1035 (2017). This review discusses the current stage of precision cancer and suggests future applications.
Le Tourneau, C. et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 16, 1324–1334 (2015).
Meric-Bernstam, F. et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J. Clin. Oncol. 33, 2753–2762 (2015).
Sholl, L. M. et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight https://doi.org/10.1172/jci.insight.87062 (2016).
Schwaederle, M. et al. On the road to precision cancer medicine: analysis of genomic biomarker actionability in 439 patients. Mol. Cancer Ther. 14, 1488–1494 (2015).
Flaherty, K. T. et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer institute molecular analysis for therapy choice (NCI-MATCH). J. Clin. Oncol. 38, 3883–3894 (2020).
Murciano-Goroff, Y. R., Taylor, B. S., Hyman, D. M. & Schram, A. M. Toward a more precise future for oncology. Cancer Cell 37, 431–442 (2020).
Murciano-Goroff, Y. R., Drilon, A. & Stadler, Z. K. The NCI-MATCH: a national, collaborative precision oncology trial for diverse tumor histologies. Cancer Cell 39, 22–24 (2021).
Friedman, A. A., Letai, A., Fisher, D. E. & Flaherty, K. T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Xu, R., Zhou, X., Wang, S. & Trinkle, C. Tumor organoid models in precision medicine and investigating cancer–stromal interactions. Pharmacol. Ther. 218, 107668 (2021).
Kondo, T. Current status and perspectives of patient-derived rare cancer models. Hum. Cell 33, 919–929 (2020).
Kawasaki, K. et al. An organoid biobank of neuroendocrine neoplasms enables genotype-phenotype mapping. Cell 183, 1420–1435.e21 (2020).
Guillen, K. P. et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat. Cancer 3, 232–250 (2022).
Hubert, C. G. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76, 2465–2477 (2016).
Matano, M. et al. Modeling colorectal cancer using CRISPR–Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Ettayebi, K. et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393 (2016).
Heo, I. et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 3, 814–823 (2018).
Chen, S. et al. Rotavirus infection and cytopathogenesis in human biliary organoids potentially recapitulate biliary atresia development. mBio https://doi.org/10.1128/mBio.01968-20 (2020).
Sachs, N. et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 38, 100300 (2019).
Driehuis, E. et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 9, 852–871 (2019).
Wroblewski, L. E. et al. Helicobacter pylori targets cancer-associated apical–junctional constituents in gastroids and gastric epithelial cells. Gut 64, 720–730 (2015).
Co, J. Y. et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep. 26, 2509–2520.e4 (2019).
Saxena, K. et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 90, 43–56 (2016).
Salahudeen, A. A. et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 588, 670–675 (2020).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Zang, R. et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 5, 3582 (2020).
Van Goor, F. et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl Acad. Sci. USA 108, 18843–18848 (2011).
Van Goor, F. et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl Acad. Sci. USA 106, 18825–18830 (2009).
Ratjen, F. et al. Cystic fibrosis. Nat. Rev. Dis. Primers 1, 15010 (2015).
Dekkers, J. F. et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945 (2013).
Verstegen, M. M. A. et al. Human extrahepatic and intrahepatic cholangiocyte organoids show region-specific differentiation potential and model cystic fibrosis-related bile duct disease. Sci. Rep. 10, 21900 (2020).
Dekkers, J. et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 8, 344ra384 (2016).
Berkers, G. et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. 26, 1701–1708.e3 (2019).
Brewington, J. J. et al. Detection of CFTR function and modulation in primary human nasal cell spheroids. J. Cyst. Fibros. 17, 26–33 (2018).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Boye, T., Steenholdt, C., Jensen, K. & Nielsen, O. Molecular manipulations and intestinal stem cell-derived organoids in inflammatory bowel disease. Stem Cells https://doi.org/10.1093/stmcls/sxac014 (2022).
Howell, K. et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology https://doi.org/10.1053/j.gastro.2017.10.007 (2017).
Soroka, C. et al. Bile‐derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology https://doi.org/10.1002/hep.30470 (2018).
Danahay, H. et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 10, 239–252 (2015).
Seidlitz, T. et al. Mouse models of human gastric cancer subtypes with stomach-specific CreERT2-mediated pathway alterations. Gastroenterology 157, 1599–1614.e2 (2019).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e10 (2018).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Rimland, C. A. et al. Regional differences in human biliary tissues and corresponding in vitro-derived organoids. Hepatology 73, 247–267 (2021).
Sampaziotis, F. et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 23, 954–963 (2017).
Sampaziotis, F. et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 371, 839–846 (2021).
Serra, D. et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 569, 66–72 (2019).
Kozlowski, M. T., Crook, C. J. & Ku, H. T. Towards organoid culture without Matrigel. Commun. Biol. 4, 1387 (2021).
Aloia, L. et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat. Cell Biol. 21, 1321–1333 (2019).
Wang, Y. et al. A microengineered collagen scaffold for generating a polarized crypt–villus architecture of human small intestinal epithelium. Biomaterials 128, 44–55 (2017).
Lukonin, I. et al. Phenotypic landscape of intestinal organoid regeneration. Nature 586, 275–280 (2020).
Kretzschmar, K. & Clevers, H. Organoids: modeling development and the stem cell niche in a dish. Dev. Cell 38, 590–600 (2016).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Palikuqi, B. et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 585, 426–432 (2020).
Tiriac, H. et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancerpancreatic cancer organoids parallel patient response. Cancer Discov. 8, 1112–1129 (2018).
Wensink, G. E. et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 5, 30 (2021).
van Mourik, P. et al. Rationale and design of the HIT-CF organoid study: stratifying cystic fibrosis patients based on intestinal organoid response to different CFTR-modulators. Transl. Med. Commun. https://doi.org/10.1186/s41231-020-00060-3 (2020).
Foo, M. A. et al. Clinical translation of patient-derived tumour organoids — bottlenecks and strategies. Biomark Res. 10, 10 (2022).
Meier, M. A. et al. Patient-derived tumor organoids for personalized medicine in a patient with rare hepatocellular carcinoma with neuroendocrine differentiation: a case report. Commun. Med. 2, 80 (2022).
Blainey, P., Krzywinski, M. & Altman, N. Points of significance: replication. Nat. Methods 11, 879–880 (2014).
Pollard, D. A., Pollard, T. D. & Pollard, K. S. Empowering statistical methods for cellular and molecular biologists. Mol. Biol. Cell 30, 1359–1368 (2019).
Krzywinski, M. & Altman, N. Significance, P values and t-tests. Nat. Methods 10, 1041–1042 (2013).
Krzywinski, M. & Altman, N. Points of significance: error bars. Nat. Methods 10, 921–922 (2013).
Nuzzo, R. Scientific method: statistical errors. Nature 506, 150–152 (2014).
Weissgerber, T. L., Milic, N. M., Winham, S. J. & Garovic, V. D. Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol. 13, e1002128 (2015).
Lord, S. J., Velle, K. B., Mullins, R. D. & Fritz-Laylin, L. K. SuperPlots: communicating reproducibility and variability in cell biology. J. Cell Biol. https://doi.org/10.1083/jcb.202001064 (2020).
Nature. Statistics for biologists. Nature https://www.nature.com/collections/qghhqm/ (2017).
Bock, C. et al. The Organoid Cell Atlas. Nat. Biotechnol. 39, 13–17 (2021).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets — 10 years on. Nucleic Acids Res. 39, D1005–D1010 (2011).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Bredenoord, A. L., Clevers, H. & Knoblich, J. A. Human tissues in a dish: the research and ethical implications of organoid technology. Science 355, eaaf9414 (2017).
Fuhr, A., Kurtz, A., Hiepen, C. & Müller, S. Organoids as miniature twins — challenges for comparability and need for data standardization and access. Organoids 1, 28–36 (2022).
Stelzner, M. et al. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol 302, G1359–G1363 (2012).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Takebe, T. & Wells, J. M. Organoids by design. Science 364, 956–959 (2019).
Xiang, Z. et al. Long-term maintenance of mature hippocampal slices in vitro. J. Neurosci. Methods 98, 145–154 (2000).
Takebe, T., Zhang, B. & Radisic, M. Synergistic engineering: organoids meet organs-on-a-chip. Cell Stem Cell 21, 297–300 (2017).
Andersen, J. et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929.e26 (2020).
Toh, Y. C., Xing, J. & Yu, H. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials 50, 87–97 (2015).
Toh, Y. C., Blagovic, K., Yu, H. & Voldman, J. Spatially organized in vitro models instruct asymmetric stem cell differentiation. Integr. Biol. 3, 1179–1187 (2011).
Efremov, A. K. et al. Application of piconewton forces to individual filopodia reveals mechanosensory role of L-type Ca2+ channels. Biomaterials 284, 121477 (2022).
Li, Q. et al. Extracellular matrix scaffolding guides lumen elongation by inducing anisotropic intercellular mechanical tension. Nat. Cell Biol. 18, 311–318 (2016).
Abdel Fattah, A. R. et al. Actuation enhances patterning in human neural tube organoids. Nat. Commun. 12, 3192 (2021).
Yang, Q. et al. Cell fate coordinates mechano-osmotic forces in intestinal crypt formation. Nat. Cell Biol. 23, 733–744 (2021).
Rezakhani, S., Gjorevski, N. & Lutolf, M. P. Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials 276, 121020 (2021).
Zhang, C., Zhao, Z., Abdul Rahim, N. A., van Noort, D. & Yu, H. Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 9, 3185–3192 (2009).
Toh, Y. C. et al. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 7, 302–309 (2007).
Koike, H. et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut–midgut boundary. Nature 574, 112–116 (2019).
Thomas, J. D., Lee, T. & Suh, N. P. A function-based framework for understanding biological systems. Annu. Rev. Biophys. Biomol. Struct. 33, 75–93 (2004).
Ghallab, A. et al. Bile microinfarcts in cholestasis are initiated by rupture of the apical hepatocyte membrane and cause shunting of bile to sinusoidal blood. Hepatology 69, 666–683 (2019).
Zhang, Y. et al. Biomimetic niches reveal the minimal cues to trigger apical lumen formation in single hepatocytes. Nat. Mater. 19, 1026–1035 (2020).
Baptista, D., Teixeira, L., van Blitterswijk, C., Giselbrecht, S. & Truckenmuller, R. Overlooked? Underestimated? Effects of substrate curvature on cell behavior. Trends Biotechnol. 37, 838–854 (2019).
Gupta, K. et al. Actomyosin contractility drives bile regurgitation as an early response during obstructive cholestasis. J. Hepatol. 66, 1231–1240 (2017).
Grimm, D. U.S. EPA to eliminate all mammal testing by 2035. Science https://www.science.org/content/article/us-epa-eliminate-all-mammal-testing-2035 (2019).
Acknowledgements
This work was supported in part by grants from CAS (XDA16020200 to Y.A.Z.), the National Key Research, Development Program of China (2020YFA0509002 to Y.A.Z.) and the National Institutes of Health (NIH) (R01CA240718, R01CA244729 and R01CA264248 to A.So.), and funding from the Institute of Bioengineering and Bioimaging, Biomedical Research Council, Agency for Science, Technology and Research (A*STAR) (Project Number IAF (H18/01/a0/017)), SMART CAMP, The Institute for Digital Medicine (WisDM) and Mechanobiology Institute of Singapore (R-714-106-004-135) to H.Y.
Ethics declarations
Competing interests
M.H. is inventor on several patents on organoid technology. A.So. and L.L. are inventors on a patent on organoid technology. A.So. is founder and owner of Icona BioDx. H.Y. is inventor on several patents on cell-based models and founder of Ants Innovate and Invitrocue. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Sebastian Merker, Toshiro Sato, Michael Schumacher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- 2.5D culture
-
3D culture with a restricted third dimension.
- Assembloids
-
Complex 3D structures combining several separately pre-generated cellular compartments and/or entities.
- Bioprinting
-
An additive manufacturing technique that enables direct deposition of stem cells, organoids and biomaterials to fabricate 3D organoid-based tissue structures with controlled cell–matrix structures.
- Engraftment rates
-
Degrees of organoid retention in a host tissue after transplantation.
- Organogenesis
-
The series of organized integrated processes that transform an amorphous mass of cells into a complete organ in the developing embryo.
- Passaging
-
The subculturing of organoids by creating either smaller organoid fragments or single cells using mechanical dissociation or enzymatic digestion.
- Stem cell niche
-
A specific tissue microenvironment where stem cells both reside and receive stimuli that regulate cell fate.
- Streptozotocin
-
An agent toxic to the insulin-producing β-cells of the pancreas in mammals.
- Temporal heterogeneity
-
Here, differences in the composition and changes during the time of passaging the organoids.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Z., Chen, X., Dowbaj, A.M. et al. Organoids. Nat Rev Methods Primers 2, 94 (2022). https://doi.org/10.1038/s43586-022-00174-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00174-y
This article is cited by
-
Development of NIR photocleavable nanoparticles with BDNF for vestibular neuron regeneration
Journal of Nanobiotechnology (2025)
-
Organoid development and applications in gynecological cancers: the new stage of tumor treatment
Journal of Nanobiotechnology (2025)
-
The application of organoids in investigating immune evasion in the microenvironment of gastric cancer and screening novel drug candidates
Molecular Cancer (2025)
-
Exploring the effects of gut microbiota on cholangiocarcinoma progression by patient-derived organoids
Journal of Translational Medicine (2025)
-
Addressing graft-versus-host disease in allogeneic cell-based immunotherapy for cancer
Experimental Hematology & Oncology (2025)