The enantioconvergent radical Suzuki-Miyaura cross-coupling of racemic alkyl halides represents a powerful approach for the construction of valuable C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} bonds. In this regard, the earth-abundant first-row transition metal (Ni, Fe, Co, and Cu) catalyst possesses a good single-electron transfer ability and can easily convert racemic alkyl halides to the prochiral alkyl radicals, providing an ideal solution for enantioconvergence. The utilization of chiral ligands would further facilitate the realization of enantioselective control over the prochiral alkyl radicals. This Perspective will discuss the advances and anticipate further development in this burgeoning field. 外消旋烷基卤化物的对映收敛自由基铃木-宫浦交叉偶联代表了构建有价值 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 键的强大方法。在这方面,地球富量的第一排过渡金属(Ni、Fe、Co 和 Cu)催化剂具有良好的单电子转移能力,能够轻松将外消旋烷基卤化物转化为原手性烷基自由基,为对映收敛提供了理想的解决方案。手性配体的利用将进一步促进对原手性烷基自由基的对映选择性控制的实现。本观点将讨论这一新兴领域的进展并展望进一步发展。
1. Introduction 1. 简介
Transition metal-catalysed Suzuki-Miyaura coupling serves as one of the most applied cross-coupling reactions to construct 过渡金属催化的铃木-宫浦偶联是应用最多的交叉偶联反应之一 ^(a){ }^{a} Department of Chemistry and Dongguan Key Laboratory for Data Science and Intelligent Medicine, Great Bay University, Dongguan 523000, China. 大湾大学化学 ^(a){ }^{a} 系、东莞市数据科学与智能医学重点实验室,辽宁 东莞 523000
E-mail: liul@gbu.edu.cn 电子邮件:liul@gbu.edu.cn ^("b "){ }^{\text {b }} Shenzhen Grubbs Institute and Department of Chemistry, Southern University of Science and Technology, Shenzhen 518055, China ^("b "){ }^{\text {b }} 南方科技大学深圳格拉布斯研究所、化学系,深圳 518055 ^(c){ }^{c} Shenzhen Key Laboratory of Cross-Coupling Reactions, Southern University of Science and Technology, Shenzhen 518055, China ^(c){ }^{c} 南方科技大学交叉偶联反应深圳重点实验室,深圳 518055 ^(d){ }^{d} Academy for Advanced Interdisciplinary Studies and Department of Chemistry, Southern University of Science and Technology, Shenzhen 518055, China 南方科技大学高等交叉学科研究 ^(d){ }^{d} 院、化学系,深圳 518055
synthetically valuable C-C bonds owing to the use of stable, easily available, and low-toxic organoboron reagents. ^(1){ }^{1} As such, tremendous development has been achieved in the past several decades in the classic Suzuki-Miyaura C(sp^(2))-C\mathrm{C}\left(\mathrm{sp}^{2}\right)-\mathrm{C} coupling of (hetero)aryl/alkenyl (pseudo)halides. ^(2){ }^{2} As an analogy, the C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} coupling of alkyl (pseudo)halides has been less developed, which stems from difficult oxidative addition and facile beta\beta-H elimination of alkyl-metal complexes compared with the (hetero)aryl/alkenyl-metal counterparts. ^(3){ }^{3} Notably, the achievement of asymmetric Suzuki-Miyaura C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} coupling can construct synthetically valuable enantioenriched threedimensional molecules of great interest in organic chemistry and drug synthesis. ^(4){ }^{4} In this respect, the stereospecific coup- 由于使用了稳定、容易获得和低毒的有机硼试剂,因此具有合成价值的 C-C 键。 ^(1){ }^{1} 因此,在过去的几十年里,(杂)芳基/烯基(伪)卤化物的经典铃木-宫浦 C(sp^(2))-C\mathrm{C}\left(\mathrm{sp}^{2}\right)-\mathrm{C} 偶联取得了巨大的发展。 ^(2){ }^{2} 作为类比,烷基(假)卤化物的 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 偶联尚未得到充分发展,这源于与(杂)芳基/烯基金属对应物相比,烷基金属络合物的氧化加成困难和容易 beta\beta -H 消除。 ^(3){ }^{3} 值得注意的是,不对称铃木-宫浦 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 偶联的实现可以构建具有合成价值的富集对映体的三维分子,这些分子在有机化学和药物合成中具有重要意义。 ^(4){ }^{4} 在这方面,刻板的政变——
Lin Liu 刘林
Lin Liu obtained his B.Sc. degree and Ph.D. degree from Lanzhou University in 2013 and 2018 under the supervision of Professor Yong-Qiang Tu, respectively. In 2019, he continued postdoctoral research with Professor Xin-Yuan Liu and later worked as a senior research fellow at Southern University of Science and Technology. Since 2022, he began his academic career as an assistant professor at Great Bay University. His current research interest involves transition metal-catalyzed radical transformations and asymmetric synthesis. 林柳得到了他的 B.Sc。2013 年和 2018 年分别获得兰州大学学位和博士学位,师从涂永强教授。2019 年,他继续与刘新元教授一起进行博士后研究,后在南方科技大学担任高级研究员。自 2022 年起,他在大湾大学担任助理教授,开始了他的学术生涯。他目前的研究兴趣涉及过渡金属催化的自由基转变和不对称合成。
Chang-Jiang Yang 杨江长
Chang-Jiang Yang obtained his B.Sc. degree from Hunan Normal University in 2011 and Ph.D. degree from Nankai University in 2017 under the supervision of Professor Zhengjie He. In 2018, he did his postdoctoral research with Professor Xin-Yuan Liu and then a senior research fellow in Southern University of Science and Technology until 2022. Now he is an assistant professor at Great Bay University. His research interest is the construction of carbon-based quaternary stereocenters and heteroatom stereocenters via the catalytic asymmetric approach. 杨长江获得了他的 B.Sc。2011 年获得湖南师范大学学位,2017 年获得南开大学博士学位,师从何正杰教授。2018 年,他与刘新元教授一起进行博士后研究,随后在南方科技大学担任高级研究员,直至 2022 年。现在他是大湾大学的助理教授。他的研究兴趣是通过催化不对称方法构建碳基季立体中心和杂原子立体中心。
ling of chiral alkyl electrophiles with organoboron nucleophiles using an achiral catalyst has provided an important approach for constructing the chiral C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} bonds. ^(5){ }^{5} The development of enantioconvergent C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} cross-coupling of racemic alkyl halides using chiral catalysts represents a more attractive approach since no chiral substrates are needed. ^(6){ }^{6} In this regard, the precious transition metal (Rh, Pd) catalyst has been utilized in several examples of enantioconvergent C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling via a dynamic kinetic asymmetric transformation by Fletcher and Tang, respectively. ^(7,8){ }^{7,8} 使用非手性催化剂将手性烷基亲电子试剂与有机硼亲核试剂进行检测,为构建手性 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 键提供了一种重要途径。 ^(5){ }^{5} 使用手性催化剂开发外消旋烷基卤化物的对映收敛交叉 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 偶联是一种更具吸引力的方法,因为不需要手性底物。 ^(6){ }^{6} 在这方面,贵金属(Rh、Pd)催化剂已分别通过 Fletcher 和 Tang 的动态动力学不对称变换,用于对映收敛 C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{2}\right) 偶联的几个例子中。 ^(7,8){ }^{7,8}
Compared with the precious transition metal catalyst via a two-electron insertion process, ^(7,8){ }^{7,8} the earth-abundant first-row transition metal ( Ni,Fe,Co\mathrm{Ni}, \mathrm{Fe}, \mathrm{Co}, and Cu ) catalyst possesses a good single-electron transfer (SET) ability. ^(9){ }^{9} Thus, the chiral first-row transition metal complexes can easily reduce racemic alkyl halides to smoothly generate the corresponding prochiral alkyl radicals and the oxidized transition metal complexes via an SET process. Subsequently, the interaction of the thus-oxidized transition metal complexes with the alkyl radicals could afford a single enantiomer of the coupling product. The whole process provides a good solution for the enantioconvergent radical Suzuki-Miyaura C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-C coupling of racemic alkyl halides with organoboron reagents, as pioneered by Fu and others (Scheme 1). ^(10){ }^{10} For a comparison of the first-row transition metal at the ground state, the standard electrode potentials E^(@)(M^(II)//M^(0))E^{\circ}\left(\mathrm{M}^{\mathrm{II}} / \mathrm{M}^{0}\right) are -0.44,-0.28,-0.26-0.44,-0.28,-0.26, and +0.34 V for Fe, Co , Ni, and Cu, respectively. ^(11){ }^{11} So, the SET ability follows this trend: Fe > Co∼Ni > Cu\mathrm{Fe}>\mathrm{Co} \sim \mathrm{Ni}>\mathrm{Cu}. Notably, the addition of diverse chiral ligands could greatly tune the redox potential of the first-row transition metal. This Perspective summarizes these enantioconvergent radical Suzuki-Miyaura C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} crosscoupling, which is categorized on the basis of different transition metals. In this Perspective, we will introduce the substrate scope of organoboron reagents and racemic alkyl halides, and discuss the chiral ligand development to achieve 与双电子插入工艺的贵金属过渡金属催化剂相比, ^(7,8){ }^{7,8} 地球丰度的第一排过渡金属( Ni,Fe,Co\mathrm{Ni}, \mathrm{Fe}, \mathrm{Co} 和 Cu)催化剂具有良好的单电子转移(SET)能力。 ^(9){ }^{9} 因此,手性第一排过渡金属配合物可以通过 SET 工艺轻松还原外消旋烷基卤化物,从而顺利生成相应的原手性烷基自由基和氧化过渡金属配合物。随后,氧化过渡金属络合物与烷基自由基的相互作用可以产生偶联产物的单一对映异构体。整个过程为外消旋烷基卤化物与有机硼试剂的对映收敛自由基 C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right) Suzuki-Miyaura-C 偶联提供了良好的解决方案,由 Fu 等人首创(方案 1)。 ^(10){ }^{10} 为了比较基态的第一排过渡金属,标准电极电位 E^(@)(M^(II)//M^(0))E^{\circ}\left(\mathrm{M}^{\mathrm{II}} / \mathrm{M}^{0}\right) 分别为 -0.44,-0.28,-0.26-0.44,-0.28,-0.26 、 和 +0.34 V 对于 Fe、Co、Ni 和 Cu。 ^(11){ }^{11} 所以,SET 能力遵循了这个趋势: Fe > Co∼Ni > Cu\mathrm{Fe}>\mathrm{Co} \sim \mathrm{Ni}>\mathrm{Cu} .值得注意的是,添加不同的手性配体可以极大地调节第一排过渡金属的氧化还原电位。本透视总结了这些对映收敛自由基铃木-宫浦 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 交联,并根据不同的过渡金属进行分类。在本透视中,我们将介绍有机硼试剂和外消旋烷基卤化物的底物范围,并讨论手性配体的发展以实现
Scheme 1 The first-row transition metal-catalysed enantioconvergent radical Suzuki-Miyaura coupling of racemic alkyl halides. 方案1:外消春烷基卤化物的第一排过渡金属催化对映收敛自由基铃木-宫浦偶联。
the enantioselective control. At last, we will discuss the existing challenges and anticipate continuous efforts in this emerging field. 对映选择性对照。最后,我们将讨论现有的挑战,并预计这一新兴领域的持续努力。
Compared with its congeners (palladium or platinum), nickel possesses a greater propensity to access an array of oxidation states (such as Ni^(0),Ni^(I),Ni^(II)\mathrm{Ni}^{0}, \mathrm{Ni}^{\mathrm{I}}, \mathrm{Ni}^{\mathrm{II}}, and Ni^(III)\mathrm{Ni}^{\mathrm{III}} ), which made nickel an appealing catalyst for the development of enantioconvergent radical coupling of racemic alkyl electrophiles with organoboron nucleophiles. ^(9){ }^{9} The pioneering work on Ni-catalysed enantioconvergent Suzuki-Miyaura coupling has been reported by Fu’s group in 2008, ^(12 a){ }^{12 a} showcasing C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) 与其同系物(钯或铂)相比,镍具有更大的接近一系列氧化态(例如 Ni^(0),Ni^(I),Ni^(II)\mathrm{Ni}^{0}, \mathrm{Ni}^{\mathrm{I}}, \mathrm{Ni}^{\mathrm{II}} 、 和 Ni^(III)\mathrm{Ni}^{\mathrm{III}} )的倾向,这使得镍成为开发外消旋烷基亲电子试剂与有机硼亲核试剂的对映收敛自由基偶联的有吸引力的催化剂。 ^(9){ }^{9} 2008 年,傅氏小组报道了镍催化对映收敛铃木-宫浦偶联的开创性工作, ^(12 a){ }^{12 a} 展示了 C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right)
Zhong-Liang Li 李忠良
Zhong-Liang Li obtained his B.Sc. degree from University of Science and Technology of China in 2009 and Ph.D. degree from The University of Hong Kong in 2014 under the supervision of Professor Dan Yang. In 2015, he did his postdoctoral research with Professor Xin-Yuan Liu and then a research associate professor at Southern University of Science and Technology. He now focuses on asymmetric radical reactions. 李忠良获得了他的 B.Sc。2009 年获得中国科学技术大学学位,2014 年获得香港大学博士学位,师从杨丹教授。2015 年,他与刘新元教授一起进行博士后研究,然后在南方科技大学担任研究副教授。他现在专注于不对称激进反应。
Qiang-Shuai Gu 顾强帅
Qiang-Shuai Gu completed his B.Sc. studies in 2008 from University of Science and Technology of China (USTC) and received his Ph.D. degree in 2013 from The University of Hong Kong (HKU) under the guidance of Professor Dan Yang. He continued his research career first in Professor Yang’s group at HKU and later in Professor Xin-Yuan Liu’s group at Southern University of Science and Technology (SUSTech). Now, he is a research associate professor in Academy for Advanced Interdisciplinary Studies at SUSTech. His research interests include asymmetric catalysis and asymmetric synthesis of bioactive small molecules. Qiang-Shuai Gu 完成了他的 B.Sc。2008 年毕业于中国科学技术大学,2013 年在香港大学获得博士学位,师从杨丹教授。他先是在香港大学杨教授的小组继续他的研究生涯,后来在南方科技大学刘新元教授的小组继续他的研究生涯。现在,他是南科大高级跨学科研究院的研究副教授。他的研究兴趣包括生物活性小分子的不对称催化和不对称合成。
coupling by the use of easily accessible chiral diamine ligands L^(**1)\mathbf{L}^{* 1}. In this paper, Fu and co-workers described this enantioconvergent coupling of secondary homobenzylic bromides with alkyl-(9-borabicyclo[3.3.1]nonane) (alkyl-(9-BBN)) to afford new C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) bonds with good yields and enantioselectivity (Scheme 2a). ^(12 a){ }^{12 a} Notably, rather than the use of aryl boronic acids in the racemic transformations from the same group, ^(12 b-d){ }^{12 b-d} the authors employ the highly reactive 9-BBNderived organoboron reagents as the coupling partners in the enantioconvergent transformations. After demonstrating the potential of Ni-catalysed enantioconvergent C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) coupling, the same group has developed an enantioconvergent arylations of secondary alpha\alpha-chloroamides with aryl-(9-BBN) for the formation of new C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) bonds (Scheme 2b). ^(13){ }^{13} Besides, secondary alkyl bromides bearing carbamate group or alkyl chlorides bearing proximal amine moiety could also undergo Ni-catalysed enantioconvergent C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) coupling to provide the desired chiral products bearing carbamate or amine group with excellent enantioselectivity, respectively (Scheme 2c and d). ^(14){ }^{14} Interestingly, such a catalytic system could also be smoothly accomplished with the use of directing group (including amides, carbamates, sulfonamides, and sulfones) on secondary alkyl halides to provide the C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) coupled product with good to excellent enantioselectivity (Scheme 2e and f). ^(15){ }^{15} On the basis of these reported results, the authors subsequently proposed a plausible mechanism as shown in Scheme 2g. ^(14 b,15){ }^{14 b, 15} First, the Nil ^(1)^(**)1{ }^{1}{ }^{*} \mathbf{1} complex undergoes a transmetalation process with R-(9-BBN) and gives the intermediates R-Ni^(I)L^(**)1\mathrm{R}-\mathrm{Ni}^{\mathrm{I}} \mathbf{L}^{*} \mathbf{1}. Afterward, the intermediates undergo a single electron reduction with alkyl halides to deliver prochiral alkyl radicals and the complexes R-Ni^(II)L^(**)1\mathrm{R}-\mathrm{Ni}^{\mathrm{II}} \mathbf{L}^{*} \mathbf{1} and subsequently proceed oxidative addition to give the Ni^("III ")\mathrm{Ni}^{\text {III }} intermediates. Finally, the reductive elimination of the Ni^("III ")\mathrm{Ni}^{\text {III }} intermediates delivered the desired products and regenerated Ni^(1)L^(**)1\mathrm{Ni}^{1} \mathbf{L}^{*} \mathbf{1} complexes for the next catalytic cycle. 通过使用易于获得的手性二胺配体进行偶联 L^(**1)\mathbf{L}^{* 1} 。在本文中,Fu 及其同事描述了仲高苄基溴与烷基-(9-硼联环[3.3.1]壬烷)(烷基-(9-BBN))的对映收敛偶联,以提供具有良好产率和对映选择性的新 C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) 键(方案 2a)。 ^(12 a){ }^{12 a} 值得注意的是,作者没有在同一组 ^(12 b-d){ }^{12 b-d} 的外消旋转化中使用芳基硼酸,而是使用高反应性 9-BBN 衍生的有机硼试剂作为对映收敛转化中的偶联伙伴。在证明了镍催化对映收敛偶 C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) 联的潜力后,同一小组开发了仲 alpha\alpha 氯酰胺与芳基-(9-BBN)的对映收敛芳基化以形成新 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 键(方案 2b)。 ^(13){ }^{13} 此外,含有氨基甲酸酯基团的仲烷基溴或含有近端胺部分的烷基氯化物也可以进行镍催化对映收敛偶 C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) 联,分别提供具有优异对映选择性的含有氨基甲酸酯或胺基团的手性产物(方案 2c 和 d)。 ^(14){ }^{14} 有趣的是,这种催化体系也可以通过在仲烷基卤化物上使用定向基团(包括酰胺、氨基甲酸酯、磺胺类和砜类)来顺利完成,从而为偶 C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) 联产物提供良好至优异的对映选择性(方案 2e 和 f)。 ^(15){ }^{15} 基于这些报告的结果,作者随后提出了一种合理的机制,如方案 2g 所示。 ^(14 b,15){ }^{14 b, 15} 首先,Nil ^(1)^(**)1{ }^{1}{ }^{*} \mathbf{1} 络合物与 R-(9-BBN) 经历金属转化过程,并得到中间体 R-Ni^(I)L^(**)1\mathrm{R}-\mathrm{Ni}^{\mathrm{I}} \mathbf{L}^{*} \mathbf{1} 。 之后,中间体用烷基卤化物进行单电子还原,以递送原手性烷基自由基和络合物 R-Ni^(II)L^(**)1\mathrm{R}-\mathrm{Ni}^{\mathrm{II}} \mathbf{L}^{*} \mathbf{1} ,随后进行氧化加成以得到 Ni^("III ")\mathrm{Ni}^{\text {III }} 中间体。最后,中间体的 Ni^("III ")\mathrm{Ni}^{\text {III }} 还原消除为下一个催化循环提供了所需的产物和再生 Ni^(1)L^(**)1\mathrm{Ni}^{1} \mathbf{L}^{*} \mathbf{1} 络合物。
Xin-Yuan Liu 刘鑫源
Xin-Yuan Liu obtained the B.Sc. degree from Anhui Normal University (AHNU) and Master degree from Shanghai Institute of Organic Chemistry, CAS and AHNU under the jointly supervision of Professor Shizheng Zhu and Professor Shaowu Wang. He earned his Ph.D. degree from The University of Hong Kong under supervision of Professor Chi-Ming Che in 2010. He continued postdoctoral study in the University of Hong Kong and The Scripps Research Institute. In 2012, he began his academic career at Southern University of Science and Technology and was promoted to Chair Professor in 2022. His research interest is radical asymmetric chemistry. 刘新元获得了 B.Sc。安徽师范大学(AHNU)学位和上海有机化学研究所硕士学位,师从朱世正教授和王少武教授共同指导。他于 2010 年在香港大学获得博士学位,师从车志明教授。他继续在香港大学和斯克里普斯研究所进行博士后研究。2012 年,他在南方科技大学开始了他的学术生涯,并于 2022 年晋升为讲席教授。他的研究兴趣是自由基不对称化学。
The fluorine and fluoroalkyl groups, such as fluoro-, tri-fluoromethoxy-, and trifluoromethyl moieties serve as a valuable “magic effect” in drug discovery of the pharmaceutical and agrochemical industries. ^(16){ }^{16} Based on the above development, two other groups have achieved the Ni-catalysed enantioconvergent radical Suzuki-Miyaura coupling of fluoro-, tri-fluoromethoxy-, and trifluoromethyl-substituted secondary alkyl halides, respectively. Gandelman and co-workers have established an attractive method to generate chiral fluoroalkanes with good to excellent enantioselectivity. ^(17){ }^{17} In this Ni/ chiral diamine ligand L^(**)2\mathbf{L}^{*} 2 catalytic system, diverse directing groups, including benzylic moieties, ketones, and sulfonamides in the alkyl halides are used in the cross-coupling (Scheme 3a). In 2017, Shen and co-workers developed a Ni-catalysed enantioconvergent Suzuki-Miyaura coupling of readily available alpha\alpha-bromobenzyl trifluoromethyl ethers with arylboronate lithium salts. ^(18){ }^{18} Notably, the utilization of lithium organoborate is due to its good transmetalation ability compared with the corresponding boronic acids. The ligand investigation suggested that the reaction proceeded smoothly with the use of chiral pyridine-oxazoline ligands ( L^(**)3-L^(**)6\mathbf{L}^{*} \mathbf{3}-\mathbf{L}^{*} \mathbf{6} ) and L^(**)5\mathbf{L}^{*} \mathbf{5} provided the best result in 81%81 \% yield and 88%88 \% ee. However, chiral 氟基团和氟烷基团,如氟基团、三氟甲氧基团和三氟甲基基团,在制药和农化行业的药物发现中具有宝贵的“神奇效果”。 ^(16){ }^{16} 基于上述发展,另外两个组分别实现了氟、三氟甲氧基和三氟甲基取代的仲烷基卤化物的镍催化对映收敛自由基铃木-宫浦偶联。Gandelman 及其同事建立了一种有吸引力的方法来生成具有良好至优异对映选择性的手性氟烷烃。 ^(17){ }^{17} 在这种 Ni/手性二胺配体 L^(**)2\mathbf{L}^{*} 2 催化体系中,交叉偶联中使用了不同的定向基团,包括烷基卤化物中的苄基部分、酮和磺胺类化合物(方案 3a)。2017 年,Shen 及其同事开发了一种镍催化的对映收敛铃木-宫浦偶联 alpha\alpha 的现成溴苄基三氟甲基醚与芳基硼酸锂盐。 ^(18){ }^{18} 值得注意的是,有机硼酸锂的利用是由于其与相应的硼酸相比具有良好的金属转代能力。配体研究表明,使用手性吡啶-恶唑啉配体( L^(**)3-L^(**)6\mathbf{L}^{*} \mathbf{3}-\mathbf{L}^{*} \mathbf{6} )反应进行顺利,并在 L^(**)5\mathbf{L}^{*} \mathbf{5} 产率和 88%88 \% ee 方面 81%81 \% 提供了最佳结果。然而,手性
Scheme 3 Ni-catalysed enantioconvergent radical Suzuki-Miyaura coupling to construct fluoro-, trifluoromethoxy-, and fluoroalkyl-substituted chiral centers. 方案 3 Ni 催化对映收敛自由基 Suzuki-Miyaura 偶联构建氟、三氟甲氧基和氟烷基取代的手性中心。
bisoxazoline ligands ( L^(**)7\mathbf{L}^{*} \mathbf{7} and L^(**)8\mathbf{L}^{*} \mathbf{8} ) were completely ineffective (Scheme 3b). Encouraged by the above success and used similar strategy, the same group further developed an enantioconvergent coupling of racemic fluoroalkyl-substituted benzyl halides with arylzinc reagents (in situ generated from arylboronate lithium salts with ZnBr_(2)\mathrm{ZnBr}_{2} ) to construct trifluoromethyl-, difluoro-methyl-, and monofluoromethyl-substituted chiral stereogenic centers with good to excellent enantioselectivity (Scheme 3c). ^(19){ }^{19} Recently, the Ni/chiral pyridine-oxazoline catalyst was also used in enantioconvergent coupling of racemic 3-bromo-phthalides and arylboronic acids to give chiral 3-aryl-phthalides in moderate to excellent yields with good enantioselectivity. ^(20){ }^{20} 双恶唑啉配体( L^(**)7\mathbf{L}^{*} \mathbf{7} 和 L^(**)8\mathbf{L}^{*} \mathbf{8} )完全无效(方案 3b)。受到上述成功的鼓舞并采用了类似的策略,同一小组进一步开发了外消旋氟烷基取代的卤化苄酯与芳基锌试剂(由芳基硼酸锂盐 ZnBr_(2)\mathrm{ZnBr}_{2} 原位生成)的对映收敛偶联,以构建具有良好至优异对映选择性的三氟甲基、二氟甲基和单氟甲基取代的手性立体中心(方案 3c)。 ^(19){ }^{19} 近年来,Ni/手性吡啶-恶唑啉催化剂还用于外消旋 3-溴邻苯二甲烷和芳基硼酸的对映收敛偶联,使手性 3-芳基邻苯二甲内酯具有中等至优异的收率,具有良好的对映选择性。 ^(20){ }^{20}
3. Fe-catalysed enantioconvergent radical Suzuki-Miyaura cross-coupling of racemic secondary alkyl halides 3. Fe 催化的外消旋仲烷基卤化物的对映收敛自由基铃木-宫浦交叉偶联
Iron and cobalt possess good single electron transfer ability as well, and several examples using these transition metals have been disclosed in the enantioconvergent radical cross-coup- 铁和钴也具有良好的单电子转移能力,在对映收敛自由基交叉 coup 中已经公开了几个使用这些过渡金属的实例
Scheme 4 Fe-catalysed enantioconvergent radical Suzuki-Miyaura coupling of racemic secondary alkyl halides. 方案4:外消消散仲烷基卤化物的铁催化对映收敛自由基铃木-宫浦偶联。
ling. Nakamura and co-workers have developed the first ironcatalysed enantioconvergent radical Suzuki-Miyaura coupling of tert-butyl alpha\alpha-bromopropionate with arylboronate ester lithium salts to fast access various optically active alpha\alpha-arylpropionic acids using P-stereogenic chiral bisphosphine ligand L^(**)12\mathbf{L}^{*} \mathbf{1 2} (Scheme 4a). ^(21){ }^{21} In 2020, Tyrol and co-workers have accomplished another enantioconvergent radical C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of the low reactive benzylic chlorides and arylboronic esters to obtain chiral 1,1-diarylalkanes with the use of an iron-based catalyst containing a chiral cyanobisoxazoline ligand framework Cat. 13 (Scheme 4b). ^(22){ }^{22} Although these two examples only gave a moderate to good enantioselectivity, the iron catalyst is still a considerable alternative due to its cost-effectiveness and safe properties, which might apply in pharmaceutical and agrochemical synthesis. ^(23){ }^{23} The plausible mechanism was proposed as shown in Scheme 4c. First, the Fe^(I)L^(**)\mathrm{Fe}^{\mathrm{I}} \mathbf{L}^{*} complex undergoes a single electron reduction with alkyl halides to generate prochiral alkyl radicals and the Fe^(II)L^(**)\mathrm{Fe}^{\mathrm{II}} \mathbf{L}^{*} intermediate. Afterward, the Fe^(II)L^(**)\mathrm{Fe}^{\mathrm{II}} \mathbf{L}^{*} intermediate undergoes a transmetalation process with arylboronate esters to give the aryl- Fe^("II ")L^(**)\mathrm{Fe}^{\text {II }} \mathbf{L}^{*} intermediate, which interacts with the alkyl radicals to give the aryl- Fe^("III ")L^(**)\mathrm{Fe}^{\text {III }} \mathbf{L}^{*} intermediate. The final reductive elimination step affords the desired products and regenerates the Fe^(I)L^(**)\mathrm{Fe}^{\mathrm{I}} \mathbf{L}^{*} complex. 岭。Nakamura 及其同事开发了第一个铁催化的对映收敛自由基 Suzuki-Miyaura 联用叔丁基 alpha\alpha -溴丙酸酯与芳基硼酸酯锂盐偶联,以使用 P-立体手性双膦配体 L^(**)12\mathbf{L}^{*} \mathbf{1 2} 快速获取各种光学活性 alpha\alpha 芳基丙酸(方案 4a)。 ^(21){ }^{21} 2020 年,Tyrol 及其同事使用含有手性氰基双恶唑啉配体框架 Cat 的铁基催化剂完成了低反应性苄基氯和芳基硼酸酯的另一次对映收敛自由基 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 偶联,以获得手性 1,1-二芳基烷烃。13(方案 4b)。 ^(22){ }^{22} 尽管这两个例子仅给出了中等至良好的对映选择性,但铁催化剂仍然是一个相当大的替代品,因为它具有成本效益和安全特性,可能适用于药物和农用化学品的合成。 ^(23){ }^{23} 如方案 4c 所示,提出了合理的机制。首先,络合物与 Fe^(I)L^(**)\mathrm{Fe}^{\mathrm{I}} \mathbf{L}^{*} 烷基卤化物进行单电子还原,生成原手性烷基自由基和 Fe^(II)L^(**)\mathrm{Fe}^{\mathrm{II}} \mathbf{L}^{*} 中间体。之后, Fe^(II)L^(**)\mathrm{Fe}^{\mathrm{II}} \mathbf{L}^{*} 中间体与芳基硼酸酯进行金属转代过程,得到芳基中间 Fe^("II ")L^(**)\mathrm{Fe}^{\text {II }} \mathbf{L}^{*} 体,芳基中间体与烷基自由基相互作用,得到芳基中间 Fe^("III ")L^(**)\mathrm{Fe}^{\text {III }} \mathbf{L}^{*} 体。最后的还原消除步骤提供所需的产物并再生 Fe^(I)L^(**)\mathrm{Fe}^{\mathrm{I}} \mathbf{L}^{*} 复合物。
Even though Co-catalysed cross-coupling of alkyl halides has been remarkably developed, ^(24){ }^{24} the enantioconvergent C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 尽管烷基卤化物的共催化交叉偶联已经显着发展, ^(24){ }^{24} 但对映收敛 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}
Scheme 5 Co-catalysed enantioconvergent radical Suzuki-Miyaura coupling of racemic secondary fluorinated benzyl bromides (Shen). 方案 5:外消旋仲氟化苄基溴(Shen)的共催化对映收敛自由基铃木-宫浦偶联。
cross-coupling reactions using Co catalyst have rarely been reported. ^(25){ }^{25} The major challenges lie in the lack of suitable ligands for the enantioselective control and the significant background reaction in the absence of chiral ligands. ^(24){ }^{24} Until recently, Shen and co-workers have successfully achieved the first Co\mathbf{C o} /chiral bisoxazoline ligand L^(**)19\mathbf{L}^{*} \mathbf{1 9} catalysed enantioconvergent radical Suzuki-Miyaura C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of the easily available secondary fluorinated benzyl bromides with a variety of arylboronate lithium salts in the presence of zinc bromide to give medically valuable alpha\alpha-fluorinated diarylmethane with good yields and excellent enantioselectivity (Scheme 5). ^(25 d){ }^{25 d} 使用钴催化剂的交叉偶联反应很少报道。 ^(25){ }^{25} 主要挑战在于缺乏合适的配体进行对映选择性控制,以及在没有手性配体的情况下发生显着的背景反应。 ^(24){ }^{24} 直到最近,Shen 及其同事已经成功实现了在溴化锌存在下,容易获得的仲氟化苄基溴化物与多种芳基硼酸锂盐的首个 Co\mathbf{C o} /手性双恶唑啉配体 L^(**)19\mathbf{L}^{*} \mathbf{1 9} 催化对映收敛自由基 Suzuki-Miyaura 偶 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 联,从而获得了具有良好收率和优异对映选择性的具有医学价值 alpha\alpha 的氟化二芳基甲烷(方案 5)。 ^(25 d){ }^{25 d}
5. Cu-catalysed enantioconvergent radical Suzuki-Miyaura cross-coupling of racemic alkyl halides 5. Cu 催化的外消旋烷基卤化物的对映收敛自由基铃木-宫浦交叉偶联
Compared with Ni//Fe//\mathrm{Ni} / \mathrm{Fe} / Co catalysts, copper possesses a relatively weak reducing capability, which retards the reaction initiation to generate the alkyl radicals from the corresponding alkyl halides. Thus, copper-catalysed C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} coupling of alkyl halides with organoboron reagents might undergo two pathways (the stereoablative radical process or stereospecific S_(N)2\mathrm{S}_{\mathrm{N}} 2 type process). ^(26){ }^{26} To achieve a Cu-catalysed enantioconvergent cross-coupling of racemic alkyl halides with organoboron reagents, the reaction mechanism has to completely proceed through the stereoablative radical process. To solve this challenge, we hypothesized that a rationally designed chiral elec-tron-rich ligand could enhance the reducing capability of copper, which might promote the generation of alkyl radicals via a single electron reduction of alkyl halides. As part of our continuous efforts in designing novel ligands for Cu-catalysed asymmetric radical reactions, ^(27){ }^{27} we have developed a Cu//multi\mathrm{Cu} / \mathrm{multi} dentate anionic N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P}-ligand ^(28){ }^{28} catalyst for enantioconvergent radical Sonogashira C(sp^(3))-C(sp).^(27 c)\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}(\mathrm{sp}) .{ }^{27 c} The utilization of the elec-tron-rich N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P}-ligand is crucial for the efficient radical generation and the chiral C(sp^(3))-C(sp)\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}(\mathrm{sp}) bond formation. To further prove the above hypothesis, a class of experiments on the catalysis’s redox potential was conducted, and found that the 与 Co 催化剂相比 Ni//Fe//\mathrm{Ni} / \mathrm{Fe} / ,铜的还原能力相对较弱,这阻碍了反应的引发,从而从相应的烷基卤化物中生成烷基自由基。因此,烷基卤化物与有机硼试剂的铜催 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 化偶联可能经历两种途径(立体消融自由基过程或立体特异性 S_(N)2\mathrm{S}_{\mathrm{N}} 2 类型过程)。 ^(26){ }^{26} 为了实现 Cu 催化的外消旋烷基卤化物与有机硼试剂的对映收敛交叉偶联,反应机理必须完全通过立体消融自由基过程进行。为了解决这一挑战,我们假设合理设计的手性富含电电子的配体可以增强铜的还原能力,从而通过烷基卤化物的单电子还原来促进烷基自由基的生成。作为我们不断努力设计用于铜催化不对称自由基反应的新型配体的一部分, ^(27){ }^{27} 我们开发了一种 Cu//multi\mathrm{Cu} / \mathrm{multi} 齿状阴离子 N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P} 配 ^(28){ }^{28} 体催化剂,用于对映收敛自由基 Sonogashira C(sp^(3))-C(sp).^(27 c)\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}(\mathrm{sp}) .{ }^{27 c} 富含电电子的 N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P} 配体的利用对于高效自由基生成和手性 C(sp^(3))-C(sp)\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}(\mathrm{sp}) 键的形成。为了进一步证明上述假设,对催化的氧化还原电位进行了一类实验,发现
reduction potential of Cu//N,N,P\mathrm{Cu} / \mathrm{N}, \mathrm{N}, \mathrm{P}-ligand catalyst is lower than that of Cu//\mathrm{Cu} / chiral bisoxazoline catalyst and CuBr according to the cyclic voltammogram study. The above results demonstrated that N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P}-ligand can significantly enhance the reducing capability of copper catalyst and further promote the reaction initiation process. ^(10 c,29){ }^{10 c, 29} Based on these developments, the novel catalytic system was further applied in enantioconvergent radical C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of alkyl bromides with (hetero)arylboron nucleophiles. After systematically modifying the ligand structure ( L^(**)21-L^(**)26\mathbf{L}^{*} \mathbf{2 1}-\mathbf{L}^{*} \mathbf{2 6} ), we have found that the steric bulkiness of the ligand on the ortho position of the aryl ring ( L^(**)23\mathbf{L}^{*} \mathbf{2 3} and L^(**)24\mathbf{L}^{*} \mathbf{2 4} ) could obviously improve the enantioselectivity. Notably, the reaction proceeded well with the stable and easily available neutral arylboronate esters, and the addition of ^(t)BuOLi{ }^{t} \mathrm{BuOLi} and H_(2)O\mathrm{H}_{2} \mathrm{O} can largely improve the reaction efficiency possibly due to the promotion of the transmetalation process. Furthermore, this reaction has a broad scope, tolerating the (hetero)aromatics either on the alkyl bromides or the boronate esters. Thus, it could quickly deliver pharmaceutically valuable enantioenriched 1,1-di(hetero)arylalkane, 1-aryl-1-heteroarylalkane, (hetero)aryl-benzyl alkynes as well as other chiral building blocks via follow-up transformations (Scheme 6). Notably, when using the low reactive propargyl 根据循环伏安图研究,配体催化剂的 Cu//N,N,P\mathrm{Cu} / \mathrm{N}, \mathrm{N}, \mathrm{P} 还原电位低于 Cu//\mathrm{Cu} / 手性双恶唑啉催化剂和 CuBr。上述结果表明, N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P} -配体可以显著增强铜催化剂的还原能力,进一步促进反应的引发过程。 ^(10 c,29){ }^{10 c, 29} 基于这些发展,新型催化体系进一步应用于烷基溴与(杂)芳基硼亲核试剂的对映收敛自由基 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 偶联。在系统地修饰配体结构( L^(**)21-L^(**)26\mathbf{L}^{*} \mathbf{2 1}-\mathbf{L}^{*} \mathbf{2 6} )后,我们发现配体在芳基环( L^(**)23\mathbf{L}^{*} \mathbf{2 3} 和 L^(**)24\mathbf{L}^{*} \mathbf{2 4} )的邻位上的空间体积可以明显提高对映选择性。值得注意的是,使用稳定且易于获得的中性芳基硼酸酯反应进展顺利,并且添加 ^(t)BuOLi{ }^{t} \mathrm{BuOLi} 并 H_(2)O\mathrm{H}_{2} \mathrm{O} 可大大提高反应效率,这可能是由于金属转化过程的促进。此外,该反应范围广泛,可以耐受烷基溴或硼酸酯上的(杂)芳烃。因此,它可以通过后续转化快速递送具有药用价值的富集对映体的 1,1-二(杂)芳基烷、1-芳基-1-杂芳基烷、(杂)芳基-苄基炔烃以及其他手性组成部分(方案 6)。值得注意的是,当使用低反应性炔炔基时
^(b){ }^{\mathrm{b}} With L*25 as ligand ^(b){ }^{\mathrm{b}} 以 L*25 为配体
https://cdn.mathpix.com/cropped/2025_07_23_a5ab8f1fd1b698d71a1ag-5.jpg?height=141&width=419&top_left_y=1767&top_left_x=1072
^(b) With L*25 as ligand| 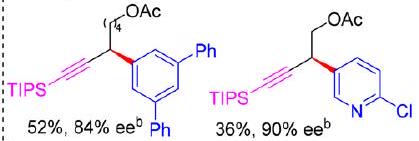 |
| :--- |
| ${ }^{\mathrm{b}}$ With L*25 as ligand |
72%, 93% ee 72%, 93% EE
54%, 82% ee 54%, 82% EE
81%, 95% ee 81%, 95% EE
89%, 95% ee 89%, 95% EE
^(a) A mixed solvent of DMSO/DCM =2//1 at -5^(@)C was used.
"CCOC(=O)C(c1ccccc1)c1cc(-c2ccccc2)cc(-c2ccccc2)c1 COc1cccc(C(C)c2ccoc2)c1 CC(c1ccsc1)c1cnc2ccccc2c1
Ph"
43%, 93% ee
"https://cdn.mathpix.com/cropped/2025_07_23_a5ab8f1fd1b698d71a1ag-5.jpg?height=141&width=419&top_left_y=1767&top_left_x=1072
^(b) With L*25 as ligand" 72%, 93% ee 54%, 82% ee
81%, 95% ee 89%, 95% ee| ${ }^{\mathrm{a}}$ A mixed solvent of DMSO/DCM $=2 / 1$ at $-5^{\circ} \mathrm{C}$ was used. | | |
| :--- | :--- | :--- |
| <smiles>CCOC(=O)C(c1ccccc1)c1cc(-c2ccccc2)cc(-c2ccccc2)c1</smiles> <smiles>COc1cccc(C(C)c2ccoc2)c1</smiles> <smiles>CC(c1ccsc1)c1cnc2ccccc2c1</smiles> <br> Ph | | |
| 43%, 93% ee | | |
|  <br> ${ }^{\mathrm{b}}$ With L*25 as ligand | 72%, 93% ee | 54%, 82% ee |
| | 81%, 95% ee | 89%, 95% ee |
Scheme 6 Cu-catalysed enantioconvergent radical Suzuki-Miyaura coupling of racemic secondary alkyl bromides with (hetero)arylboronate esters (Liu). 方案 6:Cu 催化的外消旋仲烷基溴化物与(杂)芳基硼酸酯(Liu)的对映收敛自由基 Suzuki-Miyaura 偶联。
chloride as an electrophile, this reaction could also proceed smoothly to afford the desired product with excellent enantioselectivity, but with a moderate yield. It might be due to the low reactivity of alkyl chlorides that led to the low reaction efficiency. Based on the reported results, a plausible mechanism was proposed as shown in Scheme 6. First, Cu^(I)L^(**)\mathrm{Cu}^{\mathrm{I}} \mathbf{L}^{*} complex undergoes a transmetalation process with B(mac)\mathrm{B}(\mathrm{mac}) derived arylboronate esters to give the (hetero)aryl- Cu^(I)L^(**)\mathrm{Cu}^{\mathrm{I}} \mathbf{L}^{*} intermediate. Afterward, the intermediate undergoes a single electron reduction with alkyl bromides to generate prochiral alkyl radicals and the (hetero)aryl- Cu^("II ")L^(**)\mathrm{Cu}^{\text {II }} \mathbf{L}^{*} intermediate. Finally, C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) bonds are constructed with excellent enantioselective control via the interaction of (hetero)aryl- Cu^(II)L^(**)\mathrm{Cu}^{\mathrm{II}} \mathbf{L}^{*} intermediate and alkyl radicals. ^(30){ }^{30} 氯化物作为亲电子试剂,该反应也可以顺利进行,以获得具有优异对映选择性的所需产物,但产率适中。这可能是由于烷基氯的低反应性导致反应效率低。根据报告的结果,提出了一种合理的机制,如方案 6 所示。首先, Cu^(I)L^(**)\mathrm{Cu}^{\mathrm{I}} \mathbf{L}^{*} 络合物与衍生的芳基硼酸酯进行 B(mac)\mathrm{B}(\mathrm{mac}) 金属转代过程,得到(杂)芳基中间 Cu^(I)L^(**)\mathrm{Cu}^{\mathrm{I}} \mathbf{L}^{*} 体。之后,中间体与烷基溴进行单电子还原,生成原手性烷基自由基和(杂)芳基中间 Cu^("II ")L^(**)\mathrm{Cu}^{\text {II }} \mathbf{L}^{*} 体。最后, C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 通过(杂)芳基 Cu^(II)L^(**)\mathrm{Cu}^{\mathrm{II}} \mathbf{L}^{*} 中间体和烷基自由基的相互作用,构建具有优异对映选择性控制的键。 ^(30){ }^{30}
Chiral alkenes are valuable synthons to quickly access diverse chiral building blocks, such as chiral alkanes, alcohols, aldehydes, ketones, carboxylic acids, etc. ^(31){ }^{31} As such, we next switched our attention to the enantioconvergent radical C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) cross-coupling of alkyl halides with alkenylboronate esters (Scheme 7). Unfortunately, the originally superior N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P}-ligands L^(**)24\mathbf{L}^{*} \mathbf{2 4} for (hetero)arylboronate esters showed low yields and moderate enantioselectivity in this reaction. After systematically investigating many ligand scaffolds, we discovered a hemilabile anionic N,N,N\mathrm{N}, \mathrm{N}, \mathrm{N}-ligand L^(**)27\mathbf{L}^{*} 27 to achieve the coupling of the secondary alkyl halides with alkenylboronate esters. The reaction has a broad scope, covering diverse (hetero)benzyl and propargyl bromides as well as vinyl- and mono-/disubstituted alkenylboronate esters with good to excellent yields and excellent enantioselectivity. ^(32){ }^{32} Propargyl chloride was also a suitable coupling partner for the reaction to provide the corresponding product in a diminished yield with similar ee. ^(32){ }^{32} 手性烯烃是有价值的合子,可以快速获取各种手性构件,例如手性烷烃、醇、醛、酮、羧酸等。 ^(31){ }^{31} 因此,我们接下来将注意力转向烷基卤化物与烯基硼酸酯的对映收敛自由基 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 交叉偶联(方案 7)。不幸的是,(杂)芳基硼酸酯 L^(**)24\mathbf{L}^{*} \mathbf{2 4} 的最初优 N,N,P\mathrm{N}, \mathrm{N}, \mathrm{P} 越配体在该反应中表现出低产率和中等对映选择性。在系统地研究了许多配体支架后,我们发现了一种半阴离子 N,N,N\mathrm{N}, \mathrm{N}, \mathrm{N} 配体 L^(**)27\mathbf{L}^{*} 27 ,以实现仲烷基卤化物与烯基硼酸酯的偶联。该反应范围广泛,涵盖多种(杂)苄基和炔基溴化物以及乙烯基和单/二取代烯基硼酸酯,具有良好至优异的产率和优异的对映选择性。 ^(32){ }^{32} 丙炔基氯也是该反应的合适偶联伙伴,以递减的产率提供相应的产物,并具有相似的 ee。 ^(32){ }^{32}
Encouraged by the above success of Cu-catalysed enantioconvergent C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of secondary alkyl halides with (hetero)aryl and alkenylboronate esters, we next switched our attention to the enantioconvergent radical coupling of ter- 受到铜催化仲烷基卤化物与(杂)芳基和烯基硼酸酯对映收敛偶 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 联的上述成功的鼓舞,我们接下来将注意力转向了 ter-
Scheme 8 Cu-catalysed enantioconvergent radical Suzuki-Miyaura coupling to construct alpha\alpha-quaternary beta\beta-lactams (Liu). 方案 8:Cu 催化对映收敛自由基 Suzuki-Miyaura 偶联构建 alpha\alpha -季- beta\beta 内酰胺类(Liu)。
tiary alkyl halides with these types of organoboron reagents (Scheme 8). ^(33){ }^{33} With our originally superior Cu//\mathrm{Cu} / hemilabile N,N\mathrm{N}, \mathrm{N}, N -ligand catalytic system, ^(32){ }^{32} we further successfully achieved the asymmetric cross-coupling of alpha\alpha-bromo- beta\beta-lactams with (hetero)aryl or alkenylboronate esters to construct the sterically congested quaternary stereocenters. More importantly, when allied with follow-up ring-opening reactions, this strategy could quickly deliver beta\beta-quaternary gamma\gamma-amino alcohols as well as alpha\alpha-quaternary beta\beta-amino aldehyde/esters, respectively. Different from the mechanism of our previous work, ^(30,32){ }^{30,32} this reaction may be initiated by the single-electron reduction of tertiary alkyl bromides with Cu^(I)L^(**)\mathrm{Cu}^{I} \mathbf{L}^{*}. The Cu^(I)L^(**)\mathrm{Cu}^{I} \mathbf{L}^{*} complex then undergoes the transmetalation with organoboronate esters with Cu^(II)L^(**)\mathrm{Cu}^{\mathrm{II}} \mathbf{L}^{*}, and subsequently interacts with the newly generated tertiary alkyl radicals to provide the desired chiral alpha\alpha-quaternary beta\beta-lactams. ^(34){ }^{34} 用这些类型的有机硼试剂对卤化物进行二烷基化物(方案 8)。 ^(33){ }^{33} 利用我们原本优越的 Cu//\mathrm{Cu} / 半-配体 N,N\mathrm{N}, \mathrm{N} 、N-配体催化体系, ^(32){ }^{32} 进一步成功实现 alpha\alpha 了-溴- beta\beta -内酰胺与(杂)芳基或烯基硼酸酯的不对称交叉偶联,构建了空间位充血的四元立体中心。更重要的是,当与后续的开环反应结合时,该策略可以分别快速递送 beta\beta -季- gamma\gamma 氨基醇和 alpha\alpha -季- beta\beta 氨基醛/酯。与我们之前工作的机制不同, ^(30,32){ }^{30,32} 该反应可能是通过叔烷基溴的单电子还原引发的 Cu^(I)L^(**)\mathrm{Cu}^{I} \mathbf{L}^{*} 。然后,络 Cu^(I)L^(**)\mathrm{Cu}^{I} \mathbf{L}^{*} 合物与有机硼酸酯 Cu^(II)L^(**)\mathrm{Cu}^{\mathrm{II}} \mathbf{L}^{*} 进行金属转化,并随后与新生成的叔烷基自由基相互作用,提供所需的手性 alpha\alpha -季- beta\beta 内酰胺。 ^(34){ }^{34}
6. Conclusion and perspectives 六、结论与观点
Great efforts have been made in the development of the firstrow transition metal-catalysed enantioconvergent radical Suzuki-Miyaura couplings of racemic alkyl halides with organoboron reagents in the past two decades. Crucial to the enantioselective control is the interaction of in situ prochiral alkyl radicals generated from alkyl halides with the chiral Nu-M ^("II ")L^(**){ }^{\text {II }} \mathbf{L}^{*} complex. In this Perspective, we have summarized the recent development of Ni-catalysed C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} coupling of diverse secondary alkyl halides, Fe-catalysed C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of secondary alpha\alpha-bromo esters or benzyl halides; Cocatalysed C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of secondary fluorinated benzyl bromides, and Cu-catalysed C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) coupling of secondary benzyl/propargyl halides and tertiary alpha\alpha-bromo-beta\beta-lactams. Notably, when the less reactive alkyl chlorides were 近二十年来,外消旋烷基卤化物与有机硼试剂的第一排过渡金属催化对映收敛自由基铃木-宫浦偶联的开发做出了巨大努力。对映选择性控制的关键是烷基卤化物产生的原位原手性烷基自由基与手性 Nu-M ^("II ")L^(**){ }^{\text {II }} \mathbf{L}^{*} 络合物的相互作用。本文总结了镍催化多种仲烷基卤化物偶 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 联、铁 alpha\alpha 催化仲溴酯或苄基卤化物偶 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 联的最新发展;仲氟化苄基溴化物的共催化 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 偶 C(sp^(3))-C(sp^(2))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{2}\right) 联,以及仲苄基/炔丙基卤化物和叔 alpha\alpha -溴- beta\beta 内酰胺类的 Cu 催化偶联。值得注意的是,当反应性较低的烷基氯化物
utilized instead of the corresponding bromides as electrophiles in Ni,Fe\mathrm{Ni}, \mathrm{Fe}, and Cu catalysis, the reaction would generally afford the coupling products with diminished yields and similar ee. 在 和 Cu 催化中 Ni,Fe\mathrm{Ni}, \mathrm{Fe} 代替相应的溴化物作为亲电子试剂,该反应通常会提供产率降低且 EE 相似的偶联产物。
Despite the significant progress in this burgeoning field, there are still many challenges to be addressed and tasks to be accomplished. The scope of nucleophiles and electrophiles has to be further expanded to develop the more general and powerful enantioconvergent Suzuki-Miyaura C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} coupling. As for the scope of nucleophiles, the enantioconvergent transformation of secondary and tertiary alkyl boron reagents has not been developed probably due to the steric hindrance of these alkyl boron reagents. Despite the enormous challenges, we hope that the development of chiral ligands with low steric effect might be helpful to realize the coupling of the sterically congested alkyl organoboron reagents. As for the scope of electrophiles, most alkyl halides are limited to those generating secondary alkyl radicals with pi\pi-stabilized effect or directing group. Tertiary alkyl halides are scarcely used and only one example was reported using tertiary alpha\alpha-bromo-beta\beta-lactam. ^(31){ }^{31} The coupling of unfunctionalized alkyl halides and more tertiary alkyl halides should attract enough attention in the future. The realization of the challenging substrates calls for the design of a new catalytic cycle and the development of chiral ligands. 尽管这一新兴领域取得了重大进展,但仍有许多挑战需要解决,任务需要完成。亲核试剂和亲电子试剂的范围必须进一步扩大,以开发更通用、更强大的对映收敛铃木-宫浦 C(sp^(3))-C\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C} 耦合。至于亲核试剂的范围,仲和叔烷基硼试剂的对映收敛转化尚未发展,可能是由于这些烷基硼试剂的空间位阻。尽管面临巨大的挑战,但我们希望开发具有低空间效应的手性配体可能有助于实现空间充血的烷基有机硼试剂的偶联。至于亲电子试剂的范围,大多数烷基卤化物仅限于生成具有 pi\pi 稳定作用或定向基团的仲烷基自由基的烷基。叔烷基卤化物很少使用,只有一个使用叔 alpha\alpha 溴内 beta\beta 酰胺的例子被报道。 ^(31){ }^{31} 未官能化烷基卤化物与更多叔烷基卤化物的偶联应在未来引起足够的关注。实现具有挑战性的底物需要设计新的催化循环和手性配体的开发。
To further promote this cross-coupling development, the mechanism for the whole reaction pathway, especially the key enantio-determining step should be clearly disclosed. Thus, a series of mechanistic experiments and DFT calculations are necessary to conduct a deep understanding of the reaction process. In addition, X-ray structure analysis of chiral catalysts, the isolation of different intermediates, in situ NMR spectroscopy as well as electron paramagnetic resonance spectroscopy (EPR) analysis also contributed to the discourse of the mechanism. Finally, trace metal impurities ^(27 c,35){ }^{27 c, 35} ( Pd,Ag\mathrm{Pd}, \mathrm{Ag}, and other 3d transition metals, etc.) might be responsible for some catalytic activity in the asymmetric reaction and should be considered and investigated in future research. 为了进一步促进这种交叉偶联的发展,应明确揭示整个反应途径的机理,特别是关键的对映体决定步骤。因此,需要进行一系列机理实验和 DFT 计算,才能深入了解反应过程。此外,手性催化剂的 X 射线结构分析、不同中间体的分离、原位核磁共振波谱以及电子顺磁共振波谱(EPR)分析也为该机理的讨论做出了贡献。最后,痕量金属杂质 ^(27 c,35){ }^{27 c, 35} ( Pd,Ag\mathrm{Pd}, \mathrm{Ag} 和其他三维过渡金属等)可能是不对称反应中一些催化活性的原因,应在未来的研究中考虑和研究。
Conflicts of interest 利益冲突
There are no conflicts to declare. 没有要声明的冲突。
Acknowledgements 确认
Financial support from the National Natural Science Foundation of China (no. 22025103, 92256301, 21831002, 22201127, and 22101122), the National Key R&D Program of China (no. 2021YFF0701604 and 2021YFF0701704), Shenzhen Science and Technology Program (KQTD20210811090112004 and JCYJ20220818100604009), Shenzhen Key Laboratory of Cross-Coupling Reactions (ZDSYS20220328104200001), and Great Bay University is gratefully acknowledged. We appreciate the assistance of SUSTech Core Research Facilities. 国家自然科学基金(22025103、92256301、21831002、22201127、22101122 号)、国家重点研发计划(2021YFF0701604 和 2021YFF0701704)、深圳市科技计划(KQTD20210811090112004、JCYJ20220818100604009)、深圳交叉偶联反应重点实验室(ZDSYS20220328104200001)和大湾大学的资助。我们感谢南科大核心研究设施的帮助。
18 W. Huang, X. Wan and Q. Shen, Angew. Chem., Int. Ed., 2017, 56, 11986-11989. 18 W. Huang、X. Wan 和 Q. Shen,Angew。化学,国际版,2017,56,11986-11989。
19 W. Huang, M. Hu, X. Wan and Q. Shen, Nat. Commun., 2019, 10, 2963-2970. 19 黄伟、胡明、万徐、沈琦,国家公社,2019, 10, 2963-2970.
20 S.-Y. Xu, R. Zhang, S.-S. Zhang and C.-G. Feng, Org. Biomol. Chem., 2021, 19, 4492-4496. 20 S.-Y.徐,R. 张,S.-S.张和 C.-G.冯,组织。化学,2021,19,4492-4496。
21 (a) T. Iwamoto, C. Okuzono, L. Adak, M. Jin and M. Nakamura, Chem. Commun., 2019, 55, 1128-1131. One selected example of Fe-catalysed racemic Suzuki-Miyaura C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) coupling by Nakamura, see: (b) T. Hatakeyama, T. Hashimoto, K. K. A. D. S. Kathriarachchi, T. Zenmyo, H. Seike and M. Nakamura, Angew. Chem., Int. Ed., 2012, 51, 8834-8837. 21 (a) T. Iwamoto、C. Okuzono、L. Adak、M. Jin 和 M. Nakamura,化学公报,2019,55,1128-1131。Nakamura 的铁催化外消旋 Suzuki-Miyaura C(sp^(3))-C(sp^(3))\mathrm{C}\left(\mathrm{sp}^{3}\right)-\mathrm{C}\left(\mathrm{sp}^{3}\right) 偶联的一个选定示例,参见:(b) T. Hatakeyama、T. Hashimoto、K. K. A. D. S. Kathriarachchi、T. Zenmyo、H. Seike 和 M. Nakamura, Angew。化学,国际版,2012,51,8834-8837。