Abstract 抽象
Antler blastema progenitor cells (ABPCs) are a distinct population of skeletal mesenchymal stem cells found in regenerating deer antlers, with strong stemness and renewal capacity in vitro. Stem cell-derived extracellular vesicles (EVs) are emerging as potential therapeutic candidates that can mediate donor cells’ beneficial effects. Here, we tested the effects of ABPC-derived EVs (EVsABPC) on aging in mice and rhesus macaques (Macaca mulatta). We identified a variety of unique factors in EVsABPC and showed that in vitro, EVsABPC attenuated phenotypes of senescence in bone marrow stem cells. In aged mice and macaques, EVsABPC substantially increased femoral bone mineral density. Further, intravenous EVsABPC improved physical performance, enhanced cognitive function and reduced systemic inflammation in aged mice, while reversing epigenetic age by over 3 months. In macaques, EVABPC treatment was also neuroprotective, reduced inflammation, improved locomotor function and reduced epigenetic age by over 2 years. Our findings position ABPCs as an emerging and practical source of EVs with translational value for healthy aging interventions.
鹿茸胚瘤祖细胞 (ABPCs) 是在再生鹿茸中发现的一种独特的骨骼间充质干细胞群,在体外具有很强的干性和更新能力。干细胞衍生的细胞外囊泡 (EV) 正在成为潜在的治疗候选物,可以介导供体细胞的有益作用。在这里,我们测试了 ABPC 衍生的 EV (EVsABPC) 对小鼠和恒河猴 (Macacamulatta) 衰老的影响。我们确定了 EVsABPC 中的多种独特因子,并表明在体外,EVABPC 减弱了骨髓干细胞的衰老表型。在老年小鼠和猕猴中,EVsABPC 显着增加了股骨骨矿物质密度。此外,静脉注射 EVsABPC 改善了老年小鼠的身体机能,增强了认知功能,减少了全身炎症,同时将表观遗传年龄逆转了 3 个月以上。在猕猴中,EVABPC 治疗还具有神经保护作用,减少了炎症,改善了运动功能,并将表观遗传年龄缩短了 2 岁以上。我们的研究结果将 ABPC 定位为一种新兴且实用的 EV 来源,具有健康老龄化干预的转化价值。
Similar content being viewed by others
其他人正在查看类似内容
Main 主要
Age-related disorders represent approximately 23% of the global diseases burden1. Aging is usually viewed as an inevitable time-dependent process that impaired cellular homeostasis and repair mechanisms2,3. Aging relies on a myriad of distinct responses and several hallmarks have been established, among which, progressive alterations in intercellular communication and inter-tissue crosstalk have garnered increasing attention4,5. Recent studies on systemic factors that promote healthy aging have identified several geroprotective factors from young individuals, tissues and cells to mitigate systemic aging6,7. Notably, the restoration of aged tissues or organisms has been achieved through transfusing factors like albumin6 and tissue inhibitors of metalloproteinase 2 (ref. 7). These discoveries are driving therapeutic innovation in healthy aging, with ongoing efforts to identify emerging geroprotective factors.
年龄相关疾病约占全球疾病负担的 23%1。衰老通常被视为一个不可避免的时间依赖性过程,它会损害细胞稳态和修复机制 2,3。衰老依赖于无数不同的反应,并且已经建立了几个标志,其中,细胞间通讯和组织间串扰的进行性改变引起了越来越多的关注 4,5。最近对促进健康老龄化的系统性因素的研究已经确定了来自年轻人个体、组织和细胞的几种老年保护因子,以减轻全身性衰老 6,7。值得注意的是,衰老组织或生物体的恢复是通过输注白蛋白 6 和金属蛋白酶 2 组织抑制剂等因子实现的(参考文献 7)。这些发现正在推动健康老龄化的治疗创新,并不断努力确定新兴的老年保护因子。
Stem cells are a valuable source of geroprotective factors owing to their self-renewal and multiple differentiation nature3,8. EVs are nanosized membranous vesicles that are crucial mediators for intercellular communication by delivering components between cells9,10,11. Stem cell-derived EVs harbor geroprotective factors that can remediate aging through noncell-autonomous mechanisms12 and mirror donor cells’ therapeutic effects in preclinical/clinical studies, while avoiding the biosafety concerns of stem cells therapy9,13. Mesenchymal stem cells (MSCs) have attracted the most interest as they typically derived from postnatal tissues and have fewer safety and ethical concerns8. Yet in vitro expansion induces senescence in MSCs, compromising their geroprotective effect14. Additionally, MSCs from mammalian postnatal tissues have limited regenerative ability, restricting the efficacy of MSC-derived EVs in promoting healthy aging15. Therefore, identifying innovative MSCs with robust pro-regenerative potential might refine the role of MSC-derived EVs for geroprotective interventions.
干细胞由于其自我更新和多重分化性质,是老年保护因子的宝贵来源 3,8。EV 是纳米级膜囊泡,通过在细胞之间传递成分,成为细胞间通讯的关键介质 9,10,11。干细胞衍生的 EV 含有老年保护因子,可以通过非细胞自主机制 12 修复衰老,并在临床前/临床研究中反映供体细胞的治疗效果,同时避免干细胞疗法的生物安全问题 9,13。间充质干细胞 (MSC) 引起了人们的最大兴趣,因为它们通常来源于出生后组织,并且对安全性和伦理的担忧较少 8。然而,体外扩增会诱导 MSC 衰老,从而损害其老年保护作用 14。此外,来自哺乳动物出生后组织的 MSC 的再生能力有限,限制了 MSC 衍生的 EV 促进健康老龄化的功效 15。因此,确定具有强大促再生潜力的创新 MSC 可能会改进 MSC 衍生的 EV 在老年保护干预中的作用。
In a recent study, we discovered an innovative MSC type in regenerating deer antlers, termed ABPCs, which reside at the local antler periosteum16. Notably, ABPCs can initiate de novo antler generation, which is the only mammalian organ to fully regenerate annually in adulthood. ABPCs and their progeny can drive the growth of bony antlers at 2.75 cm per day, producing a mass of up to 15 kg and length of 120 cm within 3 months, which is the fastest organ regeneration rate observed in mammals17. Unlike conventional MSCs (bone marrow, adipose or umbilical cord origins) showing senescence after 10–15 culture cycles3,8,13,18, ABPCs maintain robust proliferative and regenerative capacities even after 50 cycles13. To our knowledge, ABPCs are the only postnatal mammalian MSCs enabling complete organ regeneration, highlighting their potential as a unique source of EVs for geroprotective therapies.
在最近的一项研究中,我们在再生鹿角中发现了一种创新的 MSC 类型,称为 ABPCs,它位于当地的鹿角骨膜 16。值得注意的是,ABPC 可以启动鹿角的从头生成,这是成年后每年唯一完全再生的哺乳动物器官。ABPCs 及其后代可以以每天 2.75 cm 的速度驱动骨鹿角的生长,在 3 个月内产生高达 15 kg 的质量和 120 cm 的长度,这是在哺乳动物中观察到的最快的器官再生速度 17。与传统的 MSC(骨髓、脂肪或脐带起源)在 10-15 个培养周期后出现衰老不同 3,8,13,18,ABPC 即使在 50 个周期后仍保持强大的增殖和再生能力 13。据我们所知,ABPC 是唯一能够实现完全器官再生的出生后哺乳动物 MSC,这凸显了它们作为老年保护疗法独特 EV 来源的潜力。
Results 结果
EVsABPC attenuate cellular senescence in vitro
EVABPC 在体外减弱细胞衰老
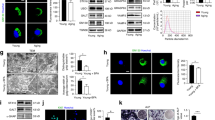
ABPCs were purified and compared to bone marrow stem cells (BMSCs) from aged (A-BMSCs) and fetal (F-BMSCs) rats (Fig. 1a). ABPCs exhibited a higher proliferation rate, a greater proportion in the S phase and enhanced colony formation compared to BMSCs (Fig. 1b–e and Extended Data Fig. 1a–c). At 120 h, the cell count of ABPCs was 5.71-fold and 3.07-fold higher than A-BMSCs and F-BMSCs, respectively (Fig. 1b). Given that stem cells of mesenchymal origin typically undergo senescence during prolonged culture in vitro, we next evaluated senescence markers after ten consecutive passages of culture (P10). A-BMSCs displayed increased aging-related markers, including senescence-associated β-galactosidase (SA-β-gal), p21 and γ-H2AX and adipocyte differentiation tendency. In contrast, ABPCs showed the lowest senescence markers and strongest osteogenic differentiation ability (Fig. 1f–k, Extended Data Fig. 1d–f and Supplementary Fig. 1a–d). Notably, the SA-β-gal activity in ABPCs was 72.4% and 54.5% lower than that in A-BMSCs and F-BMSCs, respectively (Fig. 1f). To address potential interspecies differences in BMSCs between deer and rodent, we isolated BMSCs from young male deer (D-BMSCs) and compared them to F-BMSCs from rats. D-BMSCs and F-BMSCs exhibited similar proliferation rate, differentiation capacities and aging markers (SA-β-gal and γ-H2AX) at P10, but they differed from ABPCs (Extended Data Fig. 2a–f). Overall, ABPCs possess a superior proliferative capacity and are less prone to senescence compared to BMSCs.
纯化 ABPCs 并与来自老年 (A-BMSC) 和胎儿 (F-BMSC) 大鼠的骨髓干细胞 (BMSC) 进行比较(图 D)。 与 BMSC 相比,ABPC 表现出更高的增殖率、更大的 S 期比例和增强的集落形成(图 B)。1b–e 和扩展数据图 1a-c)。在 120 小时时,ABPCs 的细胞计数分别比 A-BMSC 和 F-BMSCs 高 5.71 倍和 3.07 倍(图 1)。 鉴于间充质来源的干细胞通常在体外长时间培养过程中经历衰老,我们接下来在连续 10 次培养后评估衰老标志物 (P10)。A-BMSCs 显示衰老相关标志物增加,包括衰老相关 β-半乳糖苷酶 (SA-β-gal) 、 p21 和 γ-H2AX 以及脂肪细胞分化倾向。相比之下,ABPCs 显示出最低的衰老标志物和最强的成骨分化能力(图 D)。1f–k,扩展数据图 1d-f 和补充图 1a-d)。值得注意的是,ABPCs 中的 SA-β-gal 活性分别比 A-BMSC 和 F-BMSCs 低 72.4% 和 54.5%(图 B)。 为了解决鹿和啮齿动物之间 BMSCs 的潜在种间差异,我们从年轻雄性鹿 (D-BMSC) 中分离了 BMSC,并将它们与大鼠的 F-BMSCs 进行了比较。D-BMSCs 和 F-BMSCs 在 P10 处表现出相似的增殖速率、分化能力和衰老标志物(SA-β-gal 和 γ-H2AX),但它们与 ABPCs 不同(扩展数据图 1)。2a-f)。总体而言,ABPC 具有卓越的增殖能力,与 BMSC 相比不易衰老。
图 1:ABPC 和 EVABPC 的生物学特性。
a, The schematic diagram for the procedures of cell collection and functional characterization of ABPCs, F-BMSCs and A-BMSCs. b, Growth curves for cells over a 120-h period of culture. Cell counts were measured every 8 h. c, The proportion of cells in different cell-cycle phase at 72 h after culture using flow cytometry. d,e, CFU-F ability (d) and the ratios of EdU-positive cells (e) after culture of different cells (n = 5). f, Representative images of SA-β-gal staining in the three groups (left) and quantitative analysis (right) (n = 5). Scale bar, 200 μm. g, Quantification of p21 positive cells in the three groups (n = 5). h, Number of γ-H2AX foci in 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei of cells (n = 5). i, Representative images of Alizarin Red staining (ARS) (left) and its quantitative analysis (right) (n = 5). Scale bar, 3 mm. j, Quantitative analysis of ALP-positive staining area (n = 5). k, Representative images of Oil Red O staining (left) and its quantitative analysis (right) (n = 5). Scale bar, 100 μm. l, Morphology of EVs detected by transmission electron microscopy. Scale bar, 100 nm. m, Schematic diagram shows the transcriptomic and proteomic analysis of EVsA-BMSC, EVsF-BMSC, and EVsABPC. n,o, GO term enrichment analysis of specifically upregulated (n) and downregulated (o) DEGs in EVsABPC compared to both EVA-BMSC and EVF-BMSC groups. p, GSEA for the enrichment of gene sets in EVsABPC compared to EVsF-BMSC. q, Scatter-plot for multi-omics analysis (transcriptome and proteome) between EVsABPC and EVsF-BMSC. The x axis represents the log2(FC) at mRNA level and the y axis represents the log2(FC) at protein level. FC, fold change. r, Network diagram represented the Metascape pathways of upregulated DEGs and DEPs in EVsABPC compared to EVsF-BMSC. Each node corresponds to a specific biological process (BP) and the size of the node is proportional to the enrichment score. Statistical significance was calculated by two-way ANOVA-Bonferroni (b) or one-way ANOVA with Bonferroni’s multiple comparisons test (c–k). Data are presented as mean ± s.d. ****P < 0.0001.
A greater abundance of vesicle-like structures was observed in ABPCs than in BMSCs, likely linked to increased EV production (Extended Data Fig. 1g). EVsABPC displayed similar morphology, surface charge and size with those of EVs derived from BMSCs (EVsBMSC) (Fig. 1l and Extended Data Figs. 1h–j and 2g–i). Additionally, EVsABPC were positive for typical EV markers, including CD9, CD81 and TSG101 (Extended Data Figs. 1k and 2j). Moreover, the concentration of EVsABPC was 2.13-, 2.20- and 9.39-fold higher than those of EVs derived from F-BMSCs (EVsF-BMSC), D-BMSCs (EVsD-BMSC) and A-BMSCs (EVsA-BMSC), respectively (Extended Data Figs. 1l and 2k), highlighting ABPCs as an attractive source for EV-based therapeutics. Next, we performed bulk RNA transcriptomic and proteomic analysis to characterize the compositions of EVs (Fig. 1m–r and Extended Data Fig. 1m–p). Compared to EVsBMSC, EVsABPC had a unique transcript profile, with upregulated differentially expressed genes (DEGs) engaged in regeneration (Vim, Rpl19 and Ybx3), proteostasis (Hspa8, Rpl11 and Hsp90aa1), telomere maintenance (Hnmpa2b1, Ctnnb1 and Tcp1) and cell-cycle regulation (Hspa8, Anp32b and Rpl26) (Fig. 1n and Extended Data Fig. 1n). In contrast, pathways associated with aging hallmarks, such as inflammation (Fcer1a, Trex1 and Cebpa) and epigenetic alterations (Zdbf2, Mtr, Apex1 and Tdrd1)19,20, were downregulated in EVsABPC (Fig. 1o). Additionally, EVsABPC outperformed EVsF-BMSC in promoting cell adhesion, motility and migration (Fig. 1p). Among the upregulated differentially expressed proteins (DEPs) in EVsABPC, key molecules such as DONSON, NDUFS2 and PARK7, are involved in the cell-cycle, oxidative stress response and inflammatory response (Extended Data Fig. 1o). Furthermore, a comprehensive multi-omics analysis, integrating both transcriptomic and proteomic data, revealed 1,979 specific DEGs (Prkar2a, Anp32b and Ftl1) and 463 specific DEPs (ADCY3, EXT1 and SPAG4) in EVsABPC compared to EVsF-BMSC (Fig. 1q). Additionally, we identified 123 shared DEGs and DEPs associated with regulation of inflammatory response (Cyld, Hspd1 and Ldlr), RNA stability (Angel2, Celf1 and Cnot1) and cell cycle (Arpp19, Cdk14 and Fbxl17)21, all of which could influence the aging process (Fig. 1q,r). Meanwhile, the downregulated DEGs or DEPs were primarily involved in the negative regulation of cell migration (Gstp1, Lrp1 and Pdgfb), neuron death (Agt, Gata3 and Ncstn) and acute inflammatory response (Fcer1a, Ptger3 and Vnn1) (Extended Data Fig. 1p). Collectively, these findings highlight the therapeutic potential of EVsABPC in mitigating age-related disorders.
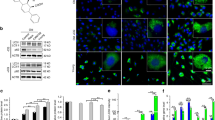
To compare the geroprotective effects of the EVs, A-BMSCs were treated daily with different EVs for three consecutive days (Fig. 2a). Notably, PKH26-labeled EVs were detected within the A-BMSCs, indicating internalization by the A-BMSCs (Fig. 2b and Extended Data Fig. 3a). Dosage-effect tests identified 8 × 108 particles per ml of EVsABPC as optimal for mitigating aging-related phenotypes and facilitating osteogenic differentiation in A-BMSCs (Extended Data Fig. 3b–d). EVsABPC were more effective in promoting cell proliferation than EVsF-BMSC and EVsA-BMSC, increasing the number of 5-ethynyl-2′-deoxyuridine (EdU)-positive cells, the proportion of S phase cells, and colony formation (Fig. 2c–f and Extended Data Fig. 3e–g). Furthermore, EVsABPC significantly lengthened telomeres (Fig. 2g) and reduced senescent markers in A-BMSCs (Fig. 2h–k, Extended Data Fig. 3h, i and Supplementary Fig. 2a,b). Notably, treatment with EVsABPC, EVsF-BMSC and EVsA-BMSC decreased the SA-β-gal activity in A-BMSCs by 57.9%, 23.6% and 10.0%, compared to the vehicle group (Fig. 2h,i). Similar effects on proliferation and aging markers were observed with EVsBMSC from both rat and deer origins (Extended Data Fig. 2l–n). Overall, these results suggest that the phenotypes of senescent BMSCs can be attenuated to varying degrees using different EVs, with EVsABPC exhibiting the strongest effect.
a, The schematic diagram for the procedure of cell culture, EVs isolation, treatment and related experiments. b, Internalization of PBS (top) and PKH26-labeled EVsABPC (bottom, yellow) in A-BMSCs stained with DAPI (blue) and phalloidin (white). Scale bar, 10 μm. c, Growth curves of A-BMSCs with different treatments for 120 h. d, Proportion of A-BMSCs in different cell-cycle phase detected after treatment with EVs for 72 h by flow cytometry. e–g, EdU-positive ratios (e), CFU-F capacity (f) and telomere lengths (g) of A-BMSCs with different treatments for 72 h (n = 5). h, Representative SA-β-gal staining of A-BMSCs treated with different EVs. Scale bar, 200 μm. i–k, The SA-β-gal-positive cell ratios (i), γ-H2AX foci cell number (j) and p21-positive cell ratios (k) of A-BMSCs. (n = 5). l–n, The proportion of positive area for ARS (l), ALP (m) and Oil Red O (n) staining in A-BMSCs (n = 5). o, PCA of the transcriptomic characteristics in A-BMSCs treated with PBS, EVsA-BMSC, EVsF-BMSC and EVsABPC, as well as PBS-treated F-BMSCs. The EVABPC-treated A-BMSCs exhibited a similar transcript profile to PBS-treated F-BMSCs, as indicated by the dashed circle. p,q, Mufzz-based clustering of DEGs in A-BMSCs treated with different EVs. Clustering trend plots showing the expression of genes across different modules (p). Heatmap of clustered genes (modules) with associated BP (q). r, GSEA of SenMayo in A-BMSCs treated with different EVs. s, Transcriptomic analysis showed that Prkar2a is the top cargo specifically in EVsABPC and abundant in EVABPC-treated cells (top). The relative expression levels of Prkar2a in EVsABPC and EVABPC-treated cells by real–time PCR (bottom). t,u, SA-β-gal staining in A-BMSCs with EVsABPC, EVs from Prkar2a-knockdown ABPCs (EVsABPC/shPrkar2a) (t), with EVsA-BMSC, EVs from Prkar2a-overexpressing A-BMSCs (EVsA-BMSC/Prkar2a) (u) (n = 5). Scale bar, 200 μm. Statistical significance was calculated by two-way ANOVA-Bonferroni (c), one-way ANOVA with Bonferroni’s multiple comparisons test (d–g and i–n) or two-tailed Student’s t-test (t,u). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Aging induces a shift in BMSCs from osteogenic to adipogenic differentiation, resulting in age-related bone loss22. EVs from both BMSCs and ABPCs reversed this process in A-BMSCs, as evidenced by increased mineralization nodule formation, alkaline phosphatase (ALP) activity, and decreased Oil Red O area (Fig. 2l–n and Extended Data Fig. 3j–l). Additionally, EVsABPC enhanced osteogenic genes expression (Alp, Runx2 and Bmp2) and suppressed adipogenic genes (Fab-4, Ppar-γ and Lpl) in A-BMSCs (Supplementary Fig. 2c,d). Among the three EVs, EVsABPC exhibited strongest effect in facilitating osteogenic differentiation, followed by EVsF-BMSC and EVsA-BMSC (Fig. 2l,m and Extended Data Fig. 3j,k). Additionally, EVsBMSC from both rat and deer origins showed minimal differences in osteogenic induction of A-BMSCs (Extended Data Fig. 2o–q). RNA-seq analysis revealed that EVsABPC altered A-BMSCs to a younger transcriptomic state, similar to F-BMSCs (Fig. 2o). Notably, the DEGs in EVABPC-treated cells possessed a unique cluster of geroprotective gene sets (Module 4), which is associated with tissue regeneration, DNA sustainment and repair, and apoptotic cells elimination (Fig. 2p,q). Subsequently, we employed a specialized gene set (SenMayo) to evaluate the senescence-associated secretory phenotype (SASP) in EV-treated cells (Supplementary Table 6)23. EVsABPC resulted in downregulation of SenMayo set in senescent BMSCs, further confirming the potential of EVsABPC in mitigating SASP in A-BMSCs (P < 0.05; Fig. 2r). Additional gene set enrichment analysis (GSEA) revealed that EVsABPC had positive effects on certain aging hallmarks, such as DNA repair and maintenance, epigenetic alterations and telomere attrition (Extended Data Fig. 4a,b), while inhibiting SASP, chronic inflammation and cell exhaustion in A-BMSCs (Extended Data Fig. 4c,d). Overall, EVsABPC demonstrated a strong geroprotective effect and restored A-BMSCs’ molecular signature to a youthful state.
To identify the key cargo within EVsABPC, we focused on messenger RNAs unique to EVsABPC but absent in EVsBMSC. A total of 2,736 mRNAs were exclusively expressed in EVsABPC, with the most abundant cargos highlighted (Fig. 2s). Given that the main function of EVs is to transfer cargos to recipient cells, we then examined the top mRNAs that were specifically upregulated in A-BMSCs treated with EVsABPC (Fig. 2s). Notably, Prkar2a, involved in the cell-cycle regulation and developmental maturation, was the only mRNA found both uniquely in EVsABPC and abundant in A-BMSCs treated with EVsABPC (Fig. 2s). This observation was further corroborated by real–time PCR (Fig. 2s). To confirm whether the high level of Prkar2a in A-BMSCs was delivered by the EVsABPC, we knocked down Prkar2a in ABPCs using Prkar2a-targeted shRNA (Extended Data Fig. 4e). Notably, EVsABPC/shPrkar2a induced compromised geroprotective effect on A-BMSCs (Fig. 2t and Extended Data Fig. 4g,h), confirming Prkar2a as a critical cargo responsible for the geroprotective effect of EVsABPC. Furthermore, elevating Prkar2a level in EVsA-BMSC enhanced their ability to attenuate phenotypes of senescence in BMSCs (Fig. 2u and Extended Data Fig. 4f,i,j), suggesting that the information gleaned from EVsABPC might enable the engineering of EVs from common sources to optimize their efficacy.
EVsABPC alleviate bone loss in aged mice
Given that ABPCs and BMSCs are skeletal-derived stem cells16, we investigated the effect of different EVs on age-related bone loss in aging mice. EVs were administered to mice every other day for 4 weeks via tail vein injection (Fig. 3a). Notably, the fluorescent dye (DiR)-labeled EVs were successfully detected in bones (Fig. 3b and Extended Data Fig. 5a). At 4 weeks, all EVs increased bone mineral density (BMD), with EVsABPC showing the greatest increase (2.5-fold of vehicle) (Fig. 3c,d). Meanwhile, EVsABPC outperformed EVsF-BMSC and EVsA-BMSC in improving trabecular bone microstructure, as evidenced by increased bone volume/total volume (BV/TV), trabecular thickness (Tb.Th) and trabecular number (Tb.N), along with decreased structure model index (SMI) and trabecular separation (Tb.Sp) (Fig. 3d); however, no significant differences were observed in cortical bones among all groups (Extended Data Fig. 5b,c). This is in line with the notion that bone loss in aged mammals predominantly occurs in trabecular bones rather than in cortical bones24. As fracture is the most common complication of bone loss24, we assessed the mechanical strength of mouse femurs using a three-point bending test. Femurs from EVABPC-treated mice exhibited the highest maximum load, yield load and Young’s modulus (Fig. 3e,f and Extended Data Fig. 5d).
a, The schematic for EV treatment in aged mice. Aged mice were treated with PBS (vehicle) or different EVs (40 μg per time), three times weekly for a duration of 4 weeks. b, Ex vivo fluorescence images (left) and average radiant efficiency (right) of femurs at 48 h after injection of DiR-labeled EVs (n = 8). Scale bar, 750 μm. c, Representative micro-CT reconstruction images of femurs from mice in different groups. Scale bar, 1,500 μm (left) or 300 μm (right) d, Quantitative analysis of BMD, BV/TV, Tb.Th, Tb.N, SMI and Tb.Sp in femurs after EV treatment (n = 8). e, Representative images of the mechanical strength of femurs in mice treated with PBS or EVsABPC. f, Quantification of the maximum load, yield load and Young’s modulus in the femurs after EVs treatment (n = 8). g,h, The serum concentration of OCN (g) and P1NP (h) post-EVs treatment (n = 8). i, Representative images of the newly formed trabecular bone in the femurs of mice using calcein AM (magenta) and Alizarin Red (white) staining. Scale bar, 50 μm. j, Quantitative analysis of MAR and BFR/BS in mice after EV treatment (n = 8). k, Representative images of ALP staining in the femurs of mice treated with PBS or EVsABPC, showing the increased number of ALP-positive cells in EVsABPC group (red arrows). Scale bar, 100 μm. l–n, Quantification of ALP (l), OCN (m) for osteoblast surfaces and TRAP (n) for osteoclast surfaces in femurs from mice with EVs treatment (n = 8). N.Ob/BS, number of osteoblasts per bone surface; N.Oc/BS, number of osteoclasts per bone surface. o,p, Representative micro-CT analysis of femurs from aged mice treated with EVsABPC, EVsABPC/shPrkar2a (o) or EVsA-BMSC, EVsA-BMSC/Prkar2a (p) (n = 8). Scale bar, 300 μm. Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (b,d,f–h,j,l–n) or two-tailed Student’s t-test (o,p). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Bone mass maintenance involves a balance between osteoblast-mediated bone formation and osteoclast-mediated resorption25. In our study, EVsABPC significantly increased serum levels of biomarkers of bone formation, osteocalcin (OCN) and procollagen type I N-terminal propeptide (P1NP), than mice in other groups (P < 0.05; Fig. 3g,h); however, the serum levels of C-telopeptide of crosslinked collagen type I (CTX-1), an indicator of bone resorption, were similar across all groups (Extended Data Fig. 5e). Additionally, EVsABPC promoted bone formation and mineralization in aged mice, with increased mineral apposition rate (MAR) and bone formation rate per bone surface area (BFR/BS) (Fig. 3i,j and Extended Data Fig. 5f). Concomitantly, EVsABPC generated a significantly more osteoblasts (ALP- or OCN-positive cells; P < 0.0001) than EVsF-BMSC and EVsA-BMSC (Fig. 3k–m and Extended Data Fig. 5g–i), whereas osteoclasts numbers, determined by tartrate resistant acid phosphatase (TRAP)-positive cells, were similar across all groups (Fig. 3n and Extended Data Fig. 5j). Collectively, these findings suggest that the beneficial effects of EVsABPC on bone loss are mainly due to enhanced osteogenesis rather than osteoclastogenesis. Considering that Prkar2a was identified as a key cargo within EVsABPC responsible for its geroprotective effect in vitro, we further investigated the effect of EVsABPC/shPrkar2a on bone loss in vivo. Knocking down Prkar2a expression in EVsABPC/shPrkar2a compromised the age-related bone loss alleviation, with a 42.7% drop in BMD compared to EVsABPC (Fig. 3o and Extended Data Fig. 5k,l), confirming the vital role of Prkar2a. Conversely, Prkar2a overexpression in EVsA-BMSC reversed bone loss in aged mice (Fig. 3p and Extended Data Fig. 5n,o). Similarly, no significance was observed in cortical bones quality among the groups (Extended Data Fig. 5m,p). These results highlight Prkar2a as a potential geroprotective factor for age-related skeletal diseases.
EVsABPC mitigate multiple aging-associated phenotypes in aged mice
Systemic administration of EVsABPC exerts broad effects, ameliorating multiple aging-associated phenotypes in aged mice. To assess EVsABPC on overall physical performance, we evaluated motor coordination and fatigue resistance using the graded-intensity rotarod tests and treadmill tests, respectively5. EV treatment improved the motor coordination (Fig. 4a) and the fatigue resistance (Fig. 4b) of mice at 14 days post-treatment, with the most notable improvements observed in the EVABPC group. These enhancements persisted for 4 weeks, peaking at day 28, when EVABPC-treated mice exhibited the most pronounced improvements in both tests (Fig. 4a,b).
a,b, Quantification of motor performance assessment by rotarod tests (a) and treadmill tests (b) (n = 8). c, SASP expression level of related inflammatory factors (IL-8, IL-6, IL-1β and TNF) in serum post-EV treatment (n = 8). d, Spearman correlation analysis was conducted to compare gene profiles in different EV-treated aged mice with ‘Young’ and ‘Old’ gene sets derived from young mice (8 weeks) and aged mice (18 months) (n = 3). Spearman’s correlation coefficient (Corr) is displayed. The fit spline (green or blue lines) and 95% confidence intervals (green or blue areas) are shown. e, Aging-related biological pathways were significantly downregulated in the EVABPC group. f, Correlation analysis of chronological age and DNAmAge for mouse blood. The Pearson’s correlation coefficient (R) is displayed (P < 0.0001). The fit spline (black line) is shown. g, The rescue of DNAmAge in aged mice after EV treatment (n = 3). Box plots display median (center line), 25th and 75th percentiles (box limits) and 1.5× interquartile range (IQR) (whiskers). h,i, Representative images of SA-β-gal staining in organs of aged mice after EVs treatment (h) and their quantitative analysis (i) (n = 8). Scale bars, 50 μm (liver, skin and brain) and 200 μm (kidney and intestine). j, Cluster analysis of upregulated DEGs specifically enriched in multi-organs with EVABPC treatment (n = 3). k,l, Spatial working memory was assessed using the Y maze as the discrimination index for the novel arm (k) and their quantitative analysis (l) (n = 8). m,n, Object recognition memory was assessed by NOR as the percentage of time exploring the novel object (m) and their quantitative analysis (n) (n = 8). o,p, Anxiety-like behavior was assessed by EPM as the percentage of open arm (o) and their quantitative analysis (p) (n = 8). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (a–c,g,i,l,n,p). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Then, we assessed the effects of the EVs on serum inflammatory markers associated with SASP, vital indicators of aging status4. All three EV types reduced the serum levels of interleukin (IL)-8, IL-6, IL-1β and tumor necrosis factor (TNF) in aged mice (P < 0.0001; Fig. 4c), with EVsABPC demonstrated the most prominent anti-inflammatory effect, followed by EVsF-BMSC and then EVsA-BMSC (Fig. 4c). This reduction suggests that EVsABPC may contribute to systemic aging amelioration. Next, we performed transcriptomic analysis of blood samples (Fig. 4d,e). Two distinct gene expression profiles were identified, termed ‘Young’ and ‘Old’, based on the top 200 DEGs between young (8-week-old) and aged (18-month-old) mice. The gene expression profile of the EVABPC-treated group correlated most strongly with the ‘Young’ set and least with the ‘Old’ set (Fig. 4d). EVsABPC also reduced gene expression associated with SASP-specific pathways (cell response to IL-1) and oxidative stress (Fig. 4e). Furthermore, we quantified the biological age through establishing a methylation clock based on blood DNA methylation profiles26. In aged mice, EVsABPC reset DNA methylation age (DNAmAge) by an average of 3.54 months, surpassing the improvements by EVsF-BMSC (0.96 months) and EVsA-BMSC (0.59 months) (Fig. 4f,g). These findings emphasize the potential of EVsABPC in reversing age-associated molecular signatures and slowing down epigenetic aging.
To systematically evaluate the geroprotective effect of EVsABPC across different tissues in aged mice, we focused on major organs, including liver, kidneys, skin, intestines and brain (Fig. 4h–j and Extended Data Fig. 6a–r). Different EVs led to a significant reduction in SA-β-gal levels in all organs, with EVsABPC exhibiting the most pronounced effect (Fig. 4h,i). In addition, EVsABPC partially reversed histological fibrosis in liver and kidneys (Extended Data Fig. 6a,b,h,i) and mitigated cell apoptosis in kidneys (Extended Data Fig. 6c,j). In the skin, EVsABPC decreased epidermal and dermal thickness, collagen content and cellular senescence (γ-H2AX) (Extended Data Fig. 6d–f,k–o). In the brain (cerebral cortex), EVsABPC provided neuroprotection, evidenced by reduced γ-H2AX foci (Extended Data Fig. 6g,p). RNA-seq revealed that EVsABPC induced distinct gene expression signatures in each organ, including collagen fibril organization in liver, DNA replication and cell-cycle regulation in kidneys, Wnt signaling in skin, collagen-activated signaling and epithelial cell proliferation in intestines, and axon ensheathment and myelination in brain (Fig. 4j and Extended Data Fig. 6q,r). Overall, EVsABPC elicit notable geroprotective effects on major organs at both the histological and molecular levels in aged mice.
The geroprotective effect of EVsABPC on brain prompted further investigation into evaluating cognitive and anxiety-like behaviors, as improvements at cellular and molecular levels in brain may not always translate to functional or behavioral improvements. Cognitive performance was assessed using two-trial Y maze and novel object recognition (NOR) task (Fig. 4k–n and Extended Data Fig. 6s,t). EVABPC-treated aged mice exhibited a stronger preference for the novel arm in the Y maze (Fig. 4k,l and Extended Data Fig. 6s) and spent 69.4% of the time exploring the novel object in the NOR task, indicating enhanced memory and object recognition (Fig. 4m,n and Extended Data Fig. 6t). Moreover, anxiety-like behaviors were evaluated using elevated plus maze (EPM). EVABPC-treated mice spent 55.2% of the time in the open arms, significantly higher than that in the other groups (Fig. 4o,p and Extended Data Fig. 6u). Additionally, EVsABPC accumulated in the brain within 48 h following tail vein injection (Extended Data Fig. 6v). These findings, combined with histological data, suggest that EVsABPC can mitigate aging-related impairments in brain function, offering potential therapeutic applications for age-related neurodegenerative diseases.
Building on our findings in male mice, we next evaluated the effects of systemic administration of EVsABPC in aged female mice. Imaging, histological and behavioral analyses demonstrated that EVABPC treatment effectively alleviated age-related bone loss and senescence phenotypes across multiple organs in female mice (Extended Data Fig. 7), aligning with the male data. These results suggest that EVsABPC exert robust geroprotective effects in both male and female mice.
EVsABPC enhance bone mass in aged rhesus macaques
To assess the translatability of the functional effects observed in mice to humans, we investigated the effect of EVsABPC on aging phenotypes in rhesus macaques27. To minimize the number of rhesus macaques in this study, only EVsF-BMSC were utilized as controls for EVsABPC, based on their superior performance over EVsA-BMSC in our previous mouse study. Nine aged female rhesus macaques (aged 16–18 years, equivalent to 56–63 years in humans) were intravenously injected with saline (n = 3), EVsF-MBSC (n = 3) and EVsABPC (n = 3) for 20 weeks (15 mg, biweekly administration for a total of ten injections), respectively. To minimize animal welfare concerns, only examinations without the need for killing animals were conducted throughout this study.
Our primary focus was the therapeutic efficacy of EVsABPC in addressing age-related bone loss (Fig. 5a). Before EV therapy, no significant differences were observed in trabecular and cortical microstructures among the groups, as examined by computed tomography (CT) (Supplementary Fig. 3a–c). After 20 weeks, both EVsABPC and EVsF-BMSC improved BMD and trabecular bone quality (Tb.Th, Tb.N and BV/TV), with EVsABPC providing superior results (Fig. 5b–d). Particularly, EVsABPC outperformed EVsF-BMSC in restoring BMD, showing a 1.53- and 1.63-fold increase in the femur and lumbar vertebrae (L5) compared to the vehicle group, respectively (Fig. 5d). Consistent with our observations in aged mice, cortical bone in aged rhesus macaques showed no significant changes following different EV treatment (Extended Data Fig. 8a,b). Furthermore, we examined the serum levels of bone metabolism markers28. Notably, bone formation indicators, such as P1NP, OCN, calcium and phosphorus levels were significantly elevated in serum by EVsABPC, but not in the EVF-BMSC group, compared to the saline group (Fig. 5e–h); however, the bone resorption indicator β-isomerized C-terminal telopeptides (β-CTX) was in the similar range across the three groups (Fig. 5i). These findings align with data in aged mice and indicate that EVsABPC can effectively ameliorate age-related bone loss in aged rhesus macaques, mainly through enhancing bone formation.
a, The schematic diagram of CT scans for the femur and lumbar vertebrae (L5) of aged rhesus macaques across groups. Aged rhesus macaques were treated with PBS (vehicle) or different EVs (15 mg per time) every two weeks for ten administrations. b, Representative 3D CT reconstruction images of the femur (top) and lumbar vertebrae (L5) (bottom). Scale bar, 5,000 μm. c, Representative 2D CT reconstruction images of the femur (left) and lumbar vertebrae (L5) (right). Scale bar, 5,000 μm (first and third columns of c), 500 μm (second and fourth columns of c). d, Quantitative analysis of the trabecular bone in femur and lumbar vertebrae (L5) from aged rhesus macaques in the three groups (n = 3). e–i, Quantitative concentration analysis of serum biomarkers, including P1NP (e), OCN (f), calcium (g), phosphorus (h) and β-CTX (i) in aged rhesus macaques post-EV treatments (n = 3). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (d–i). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
EVsABPC improve locomotor functions in aged rhesus macaques
We next examined the impact of EVsABPC on locomotor functions, which are intimately connected to musculoskeletal functions29, as observed in our mouse study. Using a three-dimensional (3D) fine-scale primate behavior analysis system30, we evaluate the movement trajectories, overall activity level per unit time and postural kinematics of rhesus macaques (Fig. 6a–e). High-speed cameras recorded 35 min of free movement and resting, tracking 14 movements and 21 sets of representative body position (Fig. 6a). No baseline differences were detected among groups (Supplementary Fig. 4a,b). After treatment, EVABPC-treated rhesus macaques spent a longer period on movements with apparent displacement (AD) (walking, turning right, turning left, looking left and head raising) than the EVF-BMSC- and saline-treated groups (Fig. 6b). No significances were observed between EVF-BMSC- and saline-treated rhesus macaques (Fig. 6b). The EVABPC group also exhibited greater activity positions diversity and faster movement speed (Fig. 6c,d), particularly in the head, back and root tail (Fig. 6e). Collectively, EVsABPC remarkably enhanced the overall locomotor activity in aged rhesus macaques in an unfamiliar environment.
a, Recording of rhesus macaques’ movements, captured by four synchronized high-speed cameras during 35 min of free movement and resting, including a total of 14 movements. b, Five movement types (walking, turning right, turning left, looking left and head raising) exhibited significant differences in the EVABPC group compared to the other groups (n = 3). c, Location heatmaps visualize the motion trajectory of 21 body parts, with different colors distinguishing various body parts. d, Speed heatmap shows the velocity of the motion trajectory projected onto a two-dimensional plane parallel to the cage bottom. e, Motion speed for three body parts (head, back and tail root) reveals significant differences among different groups (n = 3). f, The schematic diagram shows the principle of rotating Brinkman board task (top). The task involves macaques retrieving 32 food pieces from a rotating board. The board rotates clockwise at a speed of 5 rpm. The task has four stages (1) start, begins the task; (2) research, searches for food pieces; (3) grasp, grasps a food piece; and (4) pull, pulls the food piece toward itself. g, Quantification of rotating task time (top) and success rate (bottom) among different macaque groups (n = 3). h, The schematic diagram shows two states (exercise and sleep) monitored by a noninvasive monitor neck collar for 7 consecutive days. i, Quantification of total step counts in aged rhesus macaques treated with EVs during a 7-day consecutive recording period (n = 3). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (b,e,g,i). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
We next utilized a rotating Brinkman board task to evaluate monkey’s manual dexterity that indicated upper limb motor abilities31 (Fig. 6f). At baseline, the rhesus macaques exhibited the similar rotating task time and success rates (Supplementary Fig. 4c). After treatment, both EVsABPC and EVsF-BMSC improved success rates of retrievals in clockwise tasks and decreased food exploration time (Fig. 6g), with EVsABPC achieving quicker responses and higher success rates (Fig. 6g), indicating better precision grip and postural adaptation of hand in aged rhesus macaques after EVsABPC treatment. Furthermore, ActiGraph noninvasive monitor neck collar was employed to monitor the monkeys’ long-term locomotor activity within their familiar environment and living conditions, aiming to minimize potential bias associated with short-term recording (Fig. 6h). We performed consecutive monitoring of locomotor activity and sleep for 7 days in the monkey’s home cage, with no significant differences observed at baseline (Supplementary Fig. 4d,e). In the EVABPC group, aged rhesus macaques exhibited a significant increase in total steps and average steps per minute compared to other groups, consistent with the results of the 3D fine-scale primate behavioral analysis (P < 0.05; Fig. 6i and Extended Data Fig. 8c). Nevertheless, no significance was found between the groups in sleep-related indices, including daily sleep duration, time from sleep onset to awakening and the number of awakenings (Extended Data Fig. 8d–f). Our data suggest that EVABPC treatment rescue aging-induced impairments in locomotor performance, with no adverse effects on sleep in aged rhesus macaques.
EVsABPC rescue blood and bone marrow in aged rhesus macaques
We next investigated the impact of EVsABPC on serum inflammatory markers associated with SASP in aged rhesus macaques. Blood samples were collected at 20 weeks post-treatment for analysis. Both EVsABPC and EVsF-BMSC significantly decreased serum pro-inflammatory cytokines (IL-8, IL-6, IL-1β and TNF) levels (P < 0.05; Fig. 7a), with EVsABPC exhibiting a more pronounced effect (Fig. 7a), indicating their superior anti-inflammatory capacity. No significant differences in complete blood counts and serum biochemistry (liver and kidney functions) across groups were observed, confirming the safety of EV treatment (Extended Data Fig. 9a). Consistent with the mouse experiment, RNA sequencing analysis showed an increase in Prkar2a level in blood post-EVABPC treatment (Fig. 7b). Gene Ontology (GO) analysis of DEGs identified several regeneration pathways enhanced by EVsABPC, including macroautophagy, regulation of telomere maintenance, DNA replication, protein stabilization, cell-cycle checkpoint signaling and RNA stabilization (Fig. 7c). Notably, EVsABPC were more effective than EVsF-BMSC in upregulating genes in blood involved with telomere maintenance in rhesus macaques (Fig. 7d). Consistent with the findings in mice, the signs of aging delay were further supported by methylation clock analyses of blood sample32. EVsABPC reset the global epigenetic age of aged rhesus macaques by an average of 2.14 years, representing a notable improvement compared to EVsF-BMSC (Fig. 7e,f). Collectively, these results suggest that EVsABPC have a stronger capability than EVsF-BMSC to reduce serum senescence-related molecular signatures and slow the biological age rate in aged rhesus macaques.
a, Serum level of inflammatory factors of aged rhesus macaques treated with EVs (n = 3). b, Relative expression level of Prkar2a in blood samples from aged rhesus macaques by RNA-seq (n = 3). c, The biological pathways of upregulated genes in EVsF-BMSC (left) and EVsABPC (right), as compared to vehicle. d, Genes related to telomere maintenance pathway in blood samples. e, Correlation analysis of chronological age and DNAmAge for rhesus macaques’ blood. The Pearson’s correlation coefficient (R) is displayed (P < 0.0001). The fit spline (black line) is shown. f, The rescue of DNAmAge in aged rhesus macaques by EVsABPC (n = 3). Boxplots display median (center line), IQR (box limits) and 1.5 × IQR (whiskers). g–m, scRNA-seq analyzes the immune-lineage cells in bone marrow. A t-SNE plot shows the different cell types in bone marrow (n = 23,511 cells) (g). The expression levels of marker genes in seven immune-lineage cells (h). The S and G2/M phases scores across seven immune-lineage cells with different treatments. Boxplots display mean (white dot), IQR (box limits) and 1.5 × IQR (whiskers, from minimum to maximum). Lines connecting the medians across groups illustrate the overall trend of the data distribution (i). SASP gene expression strength across different cell types (j). The SASP gene expression scores across the whole immune-lineage cells. Violin plots depict the data distribution (kernel density), density (violin width), IQR (embedded box), mean (white dot) and mean ± s.d. (whiskers). Lines connecting the medians across groups illustrate the overall trend of the data distribution (k). The ratios of SASP-expressing cells across the whole immune-lineage cells (l). GSEA of three regulated pathways (m). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (a,b,f,i,k). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Aging rewires the bone marrow niche toward heightened inflammatory states with compromised osteogenesis of MSCs33. Therefore, we next proceeded to profile the responses of bone marrow cells to different EVs in aged rhesus macaques using single-cell RNA sequencing (scRNA-seq). Seven major cell subsets were identified within bone marrow immune-lineage cells (Fig. 7g,h and Extended Data Fig. 9b,c). Both EV types increased the proportion of cells in the S and G2/M phases, with EVsABPC exhibiting a more pronounced effect (P < 0.0001; Fig. 7i), suggesting the active revitalization of bone marrow cells in EVABPC-treated animals. Regarding cellular senescence, we found that EVsABPC significantly reduced the SASP level in senescent bone marrow immune cells (P < 0.0001; Fig. 7j–l), which is closely associated with aging-related bone metabolism34. Specifically, EVsABPC resulted in a greater reduction in senescent cells, in contrast to EVF-BMSC group (Fig. 7l). Additionally, strong SASP expression was enriched in the monocyte subpopulation (Extended Data Fig. 9d). Treatment with EVsABPC induced a shift in a transcriptional profile toward downregulated oxidative stress, chronic inflammation and DNA damage in the monocyte subpopulation (Fig. 7m). In contrast, EVF-BMSC-treated animals exhibited fewer benefits (Extended Data Fig. 9e). Conclusively, EVsABPC ameliorate the senescence in bone marrow immune-lineage cells, providing a beneficial niche for bone formation to counteract age-related bone loss in rhesus macaques.
EVsABPC improve brain structure in aged rhesus macaques
Based on our previous findings regarding the geroprotective effects of EVsABPC on the brain and their ability to ameliorate the overall aging status in aged rhesus macaques, we speculate that potential of EVsABPC to partially mitigate brain aging in aged rhesus macaques. Using a SPECT/CT-based imaging strategy (Fig. 8a), we observed a significant accumulation of radioactivity in the brain from 1–8 h following systemic administration of 131I-EVsABPC (Fig. 8b), which showed a high radiochemical purity of 98.3% and maintained in vitro stability of 92.0% in saline over 48 h. Notably, compared to 131I[NaI] alone, 131I-EVsABPC presented enhanced radioactive brain uptake, as evidenced by significantly higher standard uptake value (SUV) and target to background ratio (TBR) than those of adjacent background muscle (Fig. 8c,d). These findings suggest the possibility that EVs may effectively cross the blood–brain barrier, thereby highlighting their therapeutic potential in mitigating brain aging. Nevertheless, the detected cerebral radioactivity accumulation could not rule out the possibility of adherence of EVsABPC to cerebral vasculature, necessitating further investigations.
a, The schematic diagram shows using SPECT/CT to assess the situation of EVsABPC reaching the brain of aged rhesus macaques and utilizing MRI to investigate the effect of EVs on the brain structure of aged rhesus macaques. b, Biodistribution and SPECT/CT imaging of aged rhesus macaques’ brain, as indicated by dashed circles, at 1, 4 and 8 h after intravenous injection of [131I]NaI and131I-EVsABPC. c,d, Quantitative analysis of radioactive brain uptake with SUV (c) and TBR with adjacent background muscle (d) following 1, 4 and 8 h after intravenous injection of purified [131I]NaI and 131I-EVsABPC (n = 3). e–g, The percentage of changes in TIV (e), GMV (f) and WMV (g) compared to baseline over a 20-week treatment period among the groups (n = 3). h,i, The spatial brain maps for the percentage of changes in GMV (h) and WMV (i) compared to baseline, with the different colors indicating the extent of the changes in the average for each group, respectively. The x, y and z coordinates represent the 3D spatial positions within the brain, utilizing a Montreal Neurological Institute coordinate system. Statistical significance was calculated by two-way ANOVA with Bonferroni’s multiple comparisons test (c,d), or nonparametric Kruskal–Wallis test with FWE correction for multiple comparison correction followed by post hoc pairwise between-group comparison conducted by one-tailed Mann–Whitney U-tests (e–g). Data are presented as mean ± s.d. *PFWE < 0.05 and ****P < 0.0001.
Next, we conducted a noninvasive magnetic resonance imaging (MRI) to investigate the impact of EVs on the brain structure of aged rhesus macaques (Fig. 8a). Given the limited sample size in the current study, our primary objective was to identify preliminary neuroimaging phenotypes at the whole-brain level following EVABPC treatment, rather than delving into specific region-wise changes or functional exploration. After a 20-week treatment period, EVABPC administration resulted in a significant increase in total intracranial volume (TIV) (PFWE = 0.022; Fig. 8e). Specifically, this increase in TIV observed in EVABPC-treated rhesus macaques was predominantly attributed to alterations in gray matter volume (GMV), as evidenced by an elevated GMV (Fig. 8f,h), whereas white matter volume (WMV) showed no difference among the three groups (Fig. 8g,i). Furthermore, we analyzed additional morphometric properties related to gray matter to delineate the structural changes. The cortex of macaques treated with EVsABPC exhibited a tendency toward increase thickness and surface area, albeit these differences did not attain statistical significance (Extended Data Fig. 10a). This may be attributed to the small sample size or the possibility that the analysis method of voxel-based morphometry (VBM) is more sensitive in detecting structural changes while these detected brain alterations have less evident effects on surfaced-based metric such as cortical thickness and surface area35,36,37. Next, we conducted a preliminary analysis to identify potential region-specific GMV changes following EVs treatment. To balance detection sensitivity for neuroanatomical changes with the statistical limitations of our small sample size, voxel-wise comparisons were performed using a liberal statistical threshold (uncorrected P < 0.05). This analytical framework identified multiple clusters exhibiting an increased trend following EVsABPC (Extended Data Fig. 10b,c). Given our small sample size and the lack of multiple comparison correction at the voxel level, the interpretation of these findings necessitates caution and rigorous consideration; however, we posit that these data may hold promise for future studies aiming to quantitatively assess the degree of neuroprotection in brain regions most susceptible to aging or most responsive to treatment, through the enrollment of a larger cohort of animals. Additionally, no significant differences were observed in white matter metrics, including fractional anisotropy (FA) and radial diffusivity (RD), across the entire white matter region cross the three groups (Extended Data Fig. 10d). In summary, this brain imaging study provides preliminary evidence of the neuroprotective effects of EVsABPC, particularly within cerebral cortex of aged rhesus macaques.
Discussion
Our study identified ABPCs as a viable source of EVs enriched with geroprotective factors, with therapeutic benefits across multiple organs in aged mice and nonhuman primates (NHPs). EVsABPC attenuate phenotypes of senescence in stem cells, improve bone metabolism and reduce cellular senescence in bone marrow, resulting in an enhanced bone structure and strength in aged animals. Notably, EVsABPC also induced a generalized rescue in the major organs of aged male mice, resetting their epigenetic age by an average of 3.54 months. Comparable benefits were observed in female mice. In rhesus macaques, EVsABPC improve bone micro-architecture, ameliorate systemic inflammation, restore physical condition and enhance brain structure, highlighting their translational potential for clinical applications.
To date, the framework for assessing locomotor function in aging has been well established in mice, primarily employing graded-intensity treadmill and rotarod tests5; however, studies evaluating aging-related locomotor performance in aged primates remain scarce. Here, we employed a 3D fine-scale primate behavior test to assess spontaneous locomotor activity, utilizing a hierarchical 3D motion learning framework with multiple views30. This approach captures richer dynamic information compared to traditional approaches. Specifically, we placed rhesus macaques into testing cages slightly larger than their home cages within an empty room for a short-term (35 min) locomotor performance evaluation. Our analysis revealed that EVsABPC improved both movement speed and activity diversity in aged rhesus macaques. Although environmental alterations may introduce potential stress38,39, the exploratory behavior induced by this slight stress could enhance locomotion, enabling us to effectively assess the monkeys’ locomotor performance within a brief timeframe. To account for potential stress on experimental results, all testing environments and conditions were consistent, and a noninvasive neck collar monitor was used to track the long-term locomotor activity of the monkeys within their familiar environment and living conditions for 7 days. Our analysis reveals a similar locomotor improvement and good sleep quality by EVsABPC, indicating that the rescue aging-related impairments in locomotor performance were not attributes to stress. Collectively, our study provides a comprehensive understanding of locomotor changes in aged primates, and may offer valuable approaches for investigating aging-related declines in locomotor function in NHPs in future studies.
Direct delivery of geroprotective factors offers a promising strategy for delaying aging and alleviating age-related diseases. Consequently, it is crucial to identify the key cargo components within EVsABPC responsible for delaying aging and alleviating age-related diseases. Our study highlights Prkar2a as a pivotal geroprotective factor, largely recapitulates the revitalizing activity of EVsABPC in senescent cells and aging bones. Indeed, Prkar2a can translate into a component of the PKA holoenzyme, specifically the cyclic adenosine mono-phosphate (cAMP)-dependent protein kinase regulatory subunit IIα (RIIα), which decreases cell apoptosis and ameliorates inflammation40,41. Additionally, other factors within EVsABPC (Cdc20, Adra2a, Pam and Timp1) have been clarified to mitigating aging tissues and bone regeneration42. Although our focus was on mRNAs, EVs contain thousands of diverse cargos, including protein, DNAs, micro-RNAs and lipids43. Further studies should explore the relative contributions of these various components of EVsABPC to the aging process, and develop therapeutic strategies using EVsABPC and its related geroprotective factors for treating age-related diseases.
EVsABPC exhibited remarkable efficacy in ameliorating aging in multiple tissues, especially in the brain. Recent evidence has begun to shed preliminary insights into the role of EVs in brain aging. For instance, EVs isolated from the plasma of young mouse exhibit a clear cognitive-enhancing ability in aged mice by rescuing mitochondrial dysfunction12. In the current study, EVsABPC revitalized cognitive performance and reduced anxiety behaviors in aged mice. In aged rhesus macaques, long-term injection of EVsABPC exerted geroprotective effects on brain, notably increasing cerebral cortex volume. Indeed, previous studies have demonstrated that changes in mammalian brain volume and cortical-related metrics are closely associated with brain aging and function44. In a recent 40-month study, daily administration of metformin reversed brain aging by approximately 6 years in NHPs, with improvement primarily observed in cortical structure45. Considering that changes in brain structure are typically gradual, the increase in brain volume in the monkeys following 20-week treatment with EVsABPC is of translational importance. Generally, annual antler regeneration involves rapid nerve growth with a maximum rate of 2–3 cm per day, the fastest rate observed in mammals25. Additionally, EVsABPC contain various neuroprotective cargos (Nrn1, Cobl and Fgfr2), which may have contributed to the increase in brain volume. Nevertheless, future studies with a larger sample size of rhesus macaques and extended treatment duration will provide additional insights into the effect of EVsABPC on structural and functional changes in the brain, as well as identify the primary responsive brain regions.
EVsABPC offer several advantages for translational research, including a stable and abundant source of ABPCs from sika deer antlers. This makes ABPCs readily available using a standard culture and purification protocol. Additionally, ABPCs yield more EVs than MSCs at equivalent cell counts and passages. Furthermore, ABPCs can be expanded in vitro for at least 50 passages without exhibiting notable signs of senescence, whereas senescence in MSCs usually occurs after 10–15 passages. These characteristics make ABPCs a prominent source for EV-based geroprotective strategies. The utilization of EVsABPC presents a feasible strategy to overcome the limitations of stem cells, including the low retention and survival rate of stem cell delivery due to immune rejection. As EVs cannot self-replicate, concerns about tumor formation are minimized46. Additionally, EVs remain stable during long-term frozen storage or room temperature storage post-lyophilization, which is important for translational purposes47.
Although EVs are safe for application in rodents and NHPs5,27, long-term safety monitoring remains essential to exclude potential pro-tumorigenic effects. An additional safety concern regarding EVs from nonhuman species is possible adverse immunological reactions, although this was not observed in the present study. Moreover, although EVsABPC exerted geroprotective effects in female rhesus macaques, larger sample size encompassing both male and female individuals and extended longitudinal assessments are necessary for clinical translation. Furthermore, given the profound effect of EVsABPC on aging bones and organs, the use of engineering EVsABPC with tissue-specific binding peptides might allow geroprotective interventions in a specific tissue48. Finally, identifying emerging geroprotective factors that reside in EVsABPC, but not in other stem cells-derived EVs, may enable engineering of EVs from other common sources to optimize their efficacy.
Conclusively, EVsABPC provide a geroprotective strategy for attenuating phenotypes of senescence in cells and improving aging skeletons and organs in rodents and NHPs. Our study provides useful information for the development of promising treatments for age-related diseases.
Methods
Ethical approval
All animal procedures complied with Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. Studies were approved by the Animal Ethics Committee of Fourth Military Medical University (IACUC-20230075), the Institutional Animal Care and Use Committee of Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences (IACUC-PE-2023-05-002) and the Animal Ethics Committee of Changchun Sci-Tech University (CKARI202007).
Animals
Eighteen-month-old male and female healthy C57BL/6J mice were housed individually under a 12-h light–dark cycle with ad libitum access to chow and water. Animals were treated with different EVs or PBS (vehicle). The sample size (n = 8 mice per group) was determined using standard power analysis (α = 0.05, power = 0.90) with the aid of PASS (v.23.0.2). EVs (40 µg, resuspended in 100 µl PBS) or 100 µl PBS were delivered via tail vein injection three times weekly for 28 days. After the final injection, mice were killed with ketamine (87.5 mg kg−1) and xylazine (12.5 mg kg−1), followed by transcardial perfusion with ice-cold PBS and major organs were collected.
All female rhesus macaques (Macaca mulatta) (16–18 years) were housed under identical 12-h light–dark conditions, with free access to standard chow, fruit and water. Nine female macaques were involved and randomly allocated 1:1:1 into three groups (EVsABPC, EVsF-BMSC and vehicle). Over 20 weeks, each macaque received ten intravenous administrations of 15 mg EVs in 5 ml saline or only 5 ml saline. Before imaging, examinations and blood collection, animals were pre-anesthetized with atropine (0.05 mg kg−1, intramuscular injection) followed by ketamine (10 mg kg−1, intramuscular injection). Anesthesia was maintained with continuous intravenous propofol at 15 mg kg−1 h−1.
Cell isolation and culture
ABPCs were isolated as previously described using 2-year-old male sika deer16. On the fifth day post-antler shedding, animals were anesthetized, the pedicle region was sterilized, and an incision was made to expose the pedicle periosteum and regenerating antler tissue. The pedicle periosteum was collected in minimum essential medium (MEM) α for further processing. Cx43+Fgfr2+ cells were identified as ABPCs and sorted by flow cytometry. Single-cell suspensions in MEM α, 1% penicillin–streptomycin, 0.1% Mycoplasma removal agent and fetal bovine serum (FBS) were seeded in 10-cm dishes at 2 × 105 cells per cm2 and cultured. When adherent cells reached 80–90% confluence, they were passaged at the same seeding density. ABPCs at P6–P10 were used for subsequent experiments.
BMSCs were isolated from 18-month-old (A-BMSCs) and fetal (F-BMSCs) male Sprague–Dawley rats, and 2-year-old male deer (D-BMSCs). For rats, bone marrow was flushed from diaphyses using MEM α. For deer, femoral bone marrow sample was aspirated aseptically using a trocar connected to a 10-ml syringe. All BMSCs were cultured in MEM α with 10% FBS Once cells reached 80–90% confluence, adherent cells were collected and seeded at a density of 2 × 105 cells per cm2 for amplification. P6–P10 were used in all experiments.
3D cell culture
The 3D cultures were performed in 500-ml spinner flasks (CytoNiche) within a DASEA Regenbio mBioR-CC system housed in a cell culture incubator. DASEA Regencarrier Mimick microcarriers (2 g) were dispersed in 50 ml culture medium and 2.5 × 107 cells were introduced into the flask. Then final volume was adjusted to 500 ml.
Cultures underwent 48 inoculation cycles over 24 h, each comprising 5 min of stirring at 40 revolutions per minute (rpm) and 25 min resting. After 48 inoculation cycles, agitation was maintained at 40 rpm for 96 h. Culture supernatants were collected post-incubation for EV isolation.
Cell transfection
To modulate Prkar2a expression, ABPCs and A-BMSCs were transfected with lentiviruses (Genechem) encoding either Prkar2a-targeting shRNA (5′-GCATGTCTCTCCAACAATTA-3′) or Prkar2a cDNA (GenBank, NM_019264.2). Cells (1 × 105 to 1 × 106) were cultured in 12-well plates, and transfection was performed 24 h later. After 12 h, the medium was replaced with fresh complete medium. Subsequently, at 48 h post-transfection, transfection efficiency was confirmed via fluorescence microscopy and quantified by real–time PCR.
EdU assay
DNA synthesis was assessed using the Cell-Light EdU Apollo567 kit. Cells (5 × 103) were incubated with 50 μM EdU solution for 10 h. EdU-positive cells were quantified using ImageJ (v.2.14.0/1.54i).
Clonal-formation units
Cells (1 × 103) were stained with crystal violet. Colonies, identified as groups containing more than 50 cells, were counted under a light microscope.
Cell cycle analysis
Cells (1 × 106) were washed and centrifuged. Following this, the cell pellet was incubated darkly with 500 μl PI/RNase Staining Buffer for 30 min at 25 °C. Cell cycle analysis was performed by BD FACSCalibur (BD Biosciences) and ModFit LT (v.3.0).
Differentiation assays
Cells were induced in osteogenic or adipogenic medium with medium changes every 2 days for 14 and 21 days, respectively. Following differentiation, cells were stained with Alizarin Red S, ALP for osteogenic identification or Oil Red O for adipogenic differentiation. Images were photographed under a light microscope. The ratios of positive areas were determined using ImageJ.
Telomere lengths analysis
Total RNA was isolated using RIzol reagent according to the manufacturer’s instructions. Then, RNA sample (1 μg) was reverse-transcribed into complementary DNA using the ReverTra Ace qPCR RT kit. Real–time PCR was conducted using SYBR Green PCR Master Mix on a StepOnePlus real-time PCR system. Primer details are provided in Supplementary Table 5. GAPDH and β-actin served as internal control genes. Telomere lengths were normalized to 36B4 (RPLP0) as the housekeeping gene.
Isolation and characterization of EVs
Cells (2.5 × 107) were seeded in 500-ml 3D spinner flasks and cultured in medium with EV-free FBS. The supernatants were sequentially centrifuged at 750g for 20 min followed by 2,000g for 30 min. The supernatant was then centrifuged at 16,000g for 70 min. Subsequently, the supernatant was centrifuged at 100,000g for 70 min. All centrifugation was conducted at 4 °C. The resulting EV pellet was resuspended in 1 ml PBS and stored at −80 °C. Protein concentration was measured using a BCA kit.
EV characterization was confirmed by EV protein markers (CD9, CD81 and TSG101) by western blot, morphology by transmission electron microscopy and particle size by nanoparticle tracking analysis (NTA).
In vitro tracking of EV uptake
EVs were labeled with PKH26 after diluting in Diluent C at 25 °C for 5 min. After incubating with PKH26-labeled EVs at 37 °C for 24 h, cells were stained with FITC-phalloidin for 30 min and then visualized with a confocal microscope. Fluorescence intensity was quantified using ImageJ.
Ex vivo tracking of EVs
EVs (4 μg μl−1) were incubated with 15 μl DiR for 30 min at 37 °C, followed by centrifugation at 16,000g for 1 h at 4 °C. DiR-labeled EVs were injected via the tail vein in mice. At 48 h post-injection, mice were killed and organs were collected. Then fluorescence intensity was measured using the fluorescence imaging system.
Real–time PCR
Total RNA was extracted and purified from cells or EVs using the MiniBEST Universal RNA Extraction kit following the manufacturer’s instructions. Then, 2 μg of RNA was utilized for cDNA preparation employing a Transgene reverse transcription kit. The reverse transcription reaction was conducted at 16 °C for 5 min, followed by 42 °C for 60 min and concluded with a final step of 85 °C for 5 min. Real–time PCR was performed using SYBR Green Master Mix and amplified on a 7500 real-time PCR system. The PCR reaction was performed during the initial denaturation at 95 °C for 10 min, amplification for 40 cycles, denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. Expression was normalized to Gapdh using the 2C−ΔΔt method. All reactions were executed in three technical replicates. Primers are listed in Supplementary Table 5.
Histological analysis
Organs were fixed in 4% PFA for 48 h. Femurs were then decalcified in 10% EDTA for 30 days. The remaining organs were directly embedded in paraffin. Sections (8 µm) were prepared for histological and immunofluorescence staining according to standard protocols. Images were captured with a microscope and analyzed with ImageJ.
SA-β-gal assay
The level of SA-β-gal was stained by a standard kit. Cells were incubated with SA-β-gal solution for 18 h. For tissue analysis, frozen sections were incubated with SA-β-gal solution for 18 h and counterstaining with nuclear fast red. Images were acquired by light microscope, SA-β-gal-positive cells were quantified using ImageJ from randomly selected fields.
Fluorochrome calcein mineral staining
Mice were intraperitoneally injected with calcein (10 mg kg−1) and Alizarin Red (15 mg kg−1) on days 3 and 17 before killing. Femurs were collected, fixed, dehydrated and embedded in methyl methacrylate. Sections were analyzed using fluorescence microscopy. BFR/BS and MAR were calculated by ImageJ.
Immunofluorescence assay
Cells or organs were permeabilized with 0.1% Triton X-100, blocked with 10% goat serum for 2 h and incubated with primary antibodies overnight at 4 °C, followed by secondary antibody for 1 h. Finally, nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Images were captured using a fluorescence microscope and analyzed using ImageJ.
Mechanical properties
Femurs were horizontally positioned with a 9.9-mm span and tested using a Bose ELF3220 system at a displacement rate of 2 mm min−1 to generate load-displacement curves until failure. Maximum load (N), yield load (N) and Young’s modulus (MPa) were calculated.
ELISA
Mouse serum levels of OCN, P1NP, CTX-I, IL-8, IL-6, IL-1β and TNF, were measured by standard ELISA kits according to protocols.
Blood routine examination in macaques
Whole blood (1 ml) from a macaque’s femoral vein was analyzed using a XN series automated hematology analyzer.
Serum biochemical and inflammatory indicators in macaques
Blood was collected from a macaque’s femoral vein. Liver and kidney function indicators were measured using an Indiko Plus clinical and specialty chemistry analyzer. Serum levels of P1NP, OCN and β-CTX were assessed via electrochemiluminescence immunoassay. IL-6, IL-8, IL-1β and TNF were determined by chemiluminescence immunoassay. Calcium and phosphorus content were analyzed according to instructions.
Behavioral tasks in mouse
Motor coordination was assessed using a rotarod, where mice underwent a 5-min training trial at 4 rpm followed by three accelerating trials (0 to 40 rpm in 300 s) with a 1-h rest between trials. Latency to fall was recorded, with a maximum time of 300 s. Graded-intensity treadmill test was performed, starting at 10 cm s−1 for 4 min, with a 4 cm s−1 increase every 2 min until exhaustion, defined as remaining on the shock grid for over 5 s.
The Y maze test was conducted with a 5-min training session where they explored the start and trained arms while the novel arm was blocked. After a 30-min rest, mice were allowed to explore all three arms for 5 min. The discrimination index was calculated as (novel arm − trained arm)/(novel arm + trained arm), the value representing the number of entries into each arm. The NOR test was performed over 3 days: habituation (10 min), training (5 min with two identical objects A and A) and testing (5 min, one of objects A was replaced with one novel object B). The recognition index was calculated as (time spent exploring object B)/(time spent on objects A and B). The EPM test was conducted on a cross-shaped platform with two open and two closed arms. Mice explored the maze for 5 min under red light (5 lux). Anxiety-like behavior was assessed by calculating open arm/(open arm + closed arm), the value representing the number of entries into each arm. The Y maze, NOR and EPM tests were conducted after EV treatment.
Locomotor activities in macaques
A multiview video system recorded macaque spontaneous behaviors in a transparent-walled cage (150 × 150 × 150 cm) positioned centrally in a quiet room. Four Intel RealSense D435 cameras, mounted 1.1 m from the cage on pillars, captured synchronized 30-Hz footage (848 × 480 resolution) at optimized angles for full arena coverage. Subjects were transferred using a transfer box. The tests were conducted at baseline and 20 weeks after EV treatment.
Data collection and analysis utilized BehaviorAtlas NHP Explorer/Analyzer software (Bayone BioTech, v.1.01)7. A model trained on 4,200 labeled frames (21 key body parts from eight macaques) extracted 46 kinematic parameters to construct 14 movement sequences. The 21 body parts included the nose, left eye, right eye, head, neck, left shoulder, right shoulder, left elbow, right elbow, left wrist, right wrist, left limb, right limb, left knee, right knee, left ankle, right ankle, back, root tail, mid tail and tip tail. The 14 movements are in Fig. 6a. Movements were categorized as AD or without AD (NAD) and analyzed separately. Movements with NAD included hanging, crouching, standing and sitting movements and those linked to AD comprised the remaining ten movement types. Results were standardized via z-scores. Each macaque underwent two 35-min recording sessions in the morning and afternoon, respectively.
Rotating Brinkman board task
Macaques’ preferred-hand dexterity was tested over five daily sessions using a rotating Brinkman board (20-cm diameter, 5 rpm) at baseline and 20 weeks after EV treatment. Performance was scored by reward retrieval time (max 5 min) and success rate27.
Home cage activity and sleep
The ActiGraph GT9X Link (3.5 × 3.5 × 1 cm, 14 g) was attached to macaques’ collars to monitor diurnal activity (7:00–19:00) and nocturnal sleep (19:00–7:00) over 7 days. Using ActiLife 6 software (60-s epochs, v.6.13.4), locomotor activity (step counts and steps per min) and sleep parameters (total sleep time, wake after sleep onset and number of awakenings) were assessed at baseline and 20 weeks after treatment.
Micro-CT scanning
Following 4 weeks of treatment, femurs were collected, then subjected to micro-CT scanning using a Skyscan 1276 system (Bruker). The images were reconstructed by NRecon (v.1.7.3.1) and bone density data were analyzed by CRAn (v.1.18). The 3D and two-dimensional (2D) images were visualized, respectively using Dataviewer (v.0.4.8) and CTvox (v.3.3.0 r1401). The following parameters were measured: BMD, BV/TV, Tb.Th, SMI, Tb.N, Tb.Sp, cortical volume (Ct.V), cortical thickness (Ct.Th), cortical bone area (Ct.Ar) and total cortical bone area (Tt.Ar).
CT scans and analyses
High-resolution CT scans were acquired at baseline, 10 and 20 weeks after treatment by CT scanner (GE HealthCare, Lightspeed VCT). Axial imaging was performed with parameters 120 kV, 118 mA, a reconstruction diameter of 359 mm, a 512 × 512 matrix, 0.6-mm slice thickness, 0.77 × 0.77 × 0.6 mm voxel resolution and 570-mm source distance. Image reconstruction and 3D modeling were performed in 3DSlicer (v.5.6.2), with semiautomatic segmentation for regions of interest (ROIs). Femurs and lumbar vertebrae (L5) were analyzed for BMD, Tb.Th, Tb.N, BV/TV, Ct.V, Ct.Th, Cr.Ar and Tt.Ar.
SPECT/CT scans and analyses
EVsABPC were radiolabeled with 131I. Specifically, EVsABPC (100 μg in 60 μl saline) and [131I]NaI solution (2 ml, 10 mCi) were sequentially introduced into EP tubes preloaded with Iodogen (20 g), followed by purification using PD-10 columns and 0.22-μm filters. Radiolabeling efficiency was assessed from 3-μl aliquots and in vitro stability of 131I-EVsABPC in saline at 37 °C was assessed over a 48-h incubation period using instant thin-layer chromatography.
Macaques received oral Lugol’s solution (5% I2, 0.5 ml per day) for 3 days before SPECT/CT imaging. Thereafter, animals were sedated with propofol and intravenously injected with purified 131I-EVsABPC or [131I]NaI. Both the counts of empty and full needles, as well as injection times, were recorded pre- and post-injection. SPECT/CT images were performed at 1, 4 and 8 h post-injection using Symbia Inteva 6 (Siemens Healthineers). Images were acquired on a SPECT/CT dual-head gamma camera equipped with a high-energy general-purpose collimator. Whole-brain images were obtained by acquiring images for 20 s after each 3° rotation, spanning a total rotation of 180°, with a matrix size of 256 × 256 pixels. Subsequently, CT scans were conducted to generate fused SPECT/CT image. The ordered subset conjugate gradient-modified reconstruction algorithm (xSPECT Quant) was employed to determine averaged SUV and TBR, with the background SUV assessed in muscle tissue49. ROIs were delineated across the brain, and radioactive uptake activities in the brain were quantified at the various time points.
MRI data acquisition and processing
The macaques were scanned on a 3T imaging system (United Imaging Healthcare). T1-weighted anatomical images were acquired using a fast-spoiled gradient echo imaging sequence. Multi-shell diffusion-weighted images were obtained using a spin-echo planar imaging prototype sequence. Additionally, two b0 images with reversed phase-encoding were collected to correct for geometrical distortion.
The MRI structural image processing was characterized at two levels to identify morphological changes in macaques. At the volumetric level, we calculated the TIV, GMV and WMV using an SPM12 (v.r7771)-based VBM pipeline. Following DARTEL normalization (Inia19 template), tissue segmentation was performed to generate gray/white matter and cerebrospinal fluid probability maps. TIV was calculated as the sum of modulated GMV and WMV, with extracranial tissues removed through morphological mask processing. At the surface level, the brain surface was reconstructed using the HCP-NHP pipeline and cortical thickness and surface area were estimated.
The diffusion images were preprocessed using a common pipeline, which included distortion, motion and eddy current correction conducted by FMRIB Software Library (FSL, v.6.0.6.4). Preprocessed data were reconstructed by diffusion tensor imaging (DTI) models, then FA and RD were calculated (quantitative indicators widely concerned with brain aging)50,51. Whole-brain-wise FA/RD values were obtained by averaging across the FSL FAST-segmented white matter mask after affine alignment to the DTI space.
Detailed information for acquisition parameter and quality control can be found in the MRI section of the Reporting Summary.
MRI data analyses and metrics calculation
Change in each metric compared to the baseline was calculated at the whole-brain level and voxel level, respectively52.
Given the small sample size and deviation from a normal distribution, whole-brain level structural metrics were calculated. Kruskal–Wallis test for nonparametric comparisons were conducted, with Mann–Whitney U-tests (post hoc analysis) to reveal the pairwise between-group differences. Then permutation tests were performed for multiple comparisons to correct family-wise error (FWE)53: The permutation process was concurrently conducted for all pairwise comparisons among three groups. Permutation testing across three-group pairwise comparisons generated a null distribution (1,000 iterations) by retaining maximum U-values from relabeled data; true label-derived U-values determined FWE-corrected P values against this distribution.
In our voxel-wise comparison, we utilized a Kruskal–Wallis test for nonparametric comparisons to assess the voxel-wise metric across the three groups. Subsequently, Mann–Whitney U-tests were conducted as post hoc analyses to elucidate the pairwise differences between groups. Considering the relatively small sample size of our study, we did not apply multiple comparison corrections in voxel-wise level. Instead, we set a stringent significance level of P < 0.05 at the voxel level.
Bulk-seq quantification and quality control
Total RNA was extracted and sequenced using a NEBNext Ultra RNA library prep kit for Illumina with index codes for sample identification. Libraries were quantified and pooled according to effective concentrations for specific data output targets. Sequencing was performed on an Illumina platform. Raw data were processed into FASTQ format, filtered for low-quality reads with Fastp (v.0.23.1) and aligned to reference genomes (Rattus norvegicus, Mus musculus, M. mulatta and Cervus nippon). Transcript abundance was quantified using TPM via a custom script.
Proteomic analysis
Protein samples were lysed in SDT buffer (4% SDS and 10 mM Tris-HCl, pH 7.6) and protein concentrations were measured using a BCA assay kit. For each sample, 20 µg of protein was denatured and separated by SDS–PAGE (4–20% gel). Following Coomassie Blue staining, proteins were digested using filter-aided sample preparation with trypsin. Peptides were desalted and analyzed by LC–MS/MS with a TimsTOF Pro mass spectrometer coupled to a NanoElute system. Data were analyzed using MaxQuant (v.1.6.14), identifying 1,093 proteins annotated against the UniProt database.
Expression matrix analysis
Principal-component analysis (PCA) was prepared using the scatterplot3d package (v.0.3.42) in R (v.4.2.0) and DEGs/DEPs were identified using Limma (v.3.52.1) with a threshold of P < 0.05. For EVs, an absolute log2(fold change; FC) > 0.58 was used, while for peripheral blood and tissue samples, a stricter threshold (log2(FC) > 1.00) was applied. Heatmaps and clustering were visualized using TBtools-II (v.2.069) and ClusterGVis (v.0.0.9). The top 200 DEGs categorized as either upregulated or downregulated in young (8-week-old) and aged mice (18-month-old) were identified as ‘young’ and ‘old’ gene sets, respectively. Spearman’s correlation analysis was conducted to evaluate correlations between these gene sets in the various groups compared to that in the vehicle group separately for both gene sets.
Multi-omics analysis
mRNA expression from RNA-seq and protein abundance from proteomics were normalized via log2 transformation. FCs were calculated as log2 ratios of experimental to control groups, with significant changes defined by |log2(1.5)|(~0.58). Genes and proteins were categorized into four groups: mRNA + protein both, mRNA only, protein only and no change. Moderated t-statistics were used to compute P values based on linear modeling with empirical Bayes moderation. All tests were two-sided and P values were adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate method. Data visualization was carried out with a scatter-plot in ggplot2 (v.3.4.2).
DNA methylation data generation and methylation age estimation
Whole-genome bisulfite sequencing data from mice and macaques were acquired on the Illumina NovaSeq 6000 platform with paired-end 150-bp reads. Quality control ensured >30× coverage and bisulfite conversion rate >99%. Methylation levels were processed through trimming, read alignment, and methylation extraction using metratio.py. A mouse methylation age model was constructed using the GSE80672 dataset, achieving a 0.98 correlation and an average error of 1.616 months. The macaque methylation model, built from reduced representation bisulfite sequencing (NCBI SRA PRJNA610241), showed a 0.97 correlation and an average error of 1.425 years. These models were applied to predict methylation age in study samples.
ScRNA-seq and processing
Bone marrow samples from aged macaques were collected after 20 weeks of treatment (vehicle, EVsF-BMSC, EVsABPC). A 10× Chromium Controller (10× Genomics) captured 10,000 cells per sample. Libraries were constructed using a Chromium single-cell 3′ gel bead and library V3 kit, and sequencing was performed on a NovaSeq 6000 platform (Illumina). Data were aligned with Cell Ranger (v.7.0.1) to the M. mulatta genome. To remove doublets from the scRNA-seq data, the scDblFinder package (v.1.17.1) was used to identify and eliminate doublets in each sample. After filtering cells based on gene expression and mitochondrial content, 28,558 high-quality cells were retained for analysis. The filtered gene expression matrix was analyzed using the Seurat package (v.4.3.0) in R software.
To correct for technical variability, data were normalized using the Harmony package (v.0.1.1). Cell-cycle effects were assessed, and clusters were identified at a resolution of 0.3. Clustering was visualized using t-distributed stochastic neighbor embedding (t-SNE). Marker genes for each cluster were identified with a Wilcoxon rank-sum test, and cell types were annotated using SingleR. A total of 23,511 cells from seven immune cell types were used to map the aging immune microenvironment in bone marrow. Differential expression was analyzed between the vehicle and EV-treated groups.
Gene set score analysis
Gene set score analysis for the SASP was performed using the AUCell package (v.1.18.1). The SenMayo gene set (Supplementary Table 6) was used as the input gene set, and area under the curve (AUC) values were calculated using the ‘AUCell_calcAUC’ function based on gene expression rankings. AUC scores were calculated for each cell to assess senescence, with cells exhibiting an AUC > 0.0345 classified as SASP positive.
GSEA
GSEA was conducted using the clusterProfiler package (v.4.9.3), with visualization facilitated by the GseaVis package (v.0.0.9). Genes were preranked based on their differential expression significance, employing the metric, −log10(P) × (FC)/abs (FC). The Limma package was used to calculate P values and FC values. The normalized enrichment scores and P values for the pathways were computed via a one-sided permutation test (1,000 permutations) without multiple testing correction. The SenMayo gene set was specifically used to assess cellular senescence, whereas additional gene sets enriched in GO were acquired or compiled from the Molecular Signatures Database (MSigDB)23. Pathways with a minimum gene count of five and P < 0.05 were selected for subsequent analyses.
GO analysis
GO analysis of DEGs and DEPs was conducted using the Metascape (v.3.5)54 and clusterProfiler packages. Results were visualized using the ggplot2 package and Cytoscape software (v.3.8.2). The rich factor for GO terms was calculated as the count of genes in a term divided by the total number of genes in the background.
Statistics and reproducibility
All experiments were conducted with simple randomization and blinding performed by an independent researcher before EV injection. During allocation, animals were randomly assigned to each group, with group allocation being concealed from the therapists, collectors and analysts. Throughout the experiment, the therapists remained blinded to the group allocations and handled all animals uniformly. They were also unaware of the specific treatments and provided consistent care across different groups. Following treatment, data were collected by individuals who were blinded to both the treatment and group allocation. Similarly, data were analyzed by analysts who remained blinded to treatment details. Groups were unblinded after the completion of the statistical analysis. No animals or data points were excluded and omitted from the analysis. All dots in graphs represent biological replicates. Before analysis, the data were tested for normality and homoscedasticity. For data passing the normality test, two-group comparisons were performed using a Student’s t-test, whereas comparisons among more than two groups, we used one-way analysis of variance (ANOVA) for a single variable or two-way ANOVA for two variables, followed by the Bonferroni’s post hoc test. For data without normal distribution, a Mann–Whitney U-test was used for comparisons among two groups and a Kruskal–Wallis test with Dunn’s multiple comparisons was used for comparisons among more than two groups. Statistical significance was assessed using Prism (v.9.0), with significance defined as P < 0.05. Further statistical details can be found in the figure legends. Main experimental findings (such as micrographs, blots and hematology) were validated through three independently performed experiments. All replications presented similar results. All reagents or resources are listed in Supplementary Tables 1–8.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All omics data generated in this study have been deposited in the Gene Expression Omnibus database (transcriptome) and iProX database (proteome), which have been publicly released. The transcriptomic data of EVs, cells and mice (including serum and tissues) are accessible via GSE289038. The transcriptomic data of rhesus macaques are accessible via GSE273278. For scRNA-seq of rhesus macaques, the data are accessible via GSE288582. For proteomics of EVs, the data are accessible via PXD064039. Additionally, all data associated with this study are present in the paper or the Supplementary Information. Source data are provided with the paper.
Code availability
The code used in this study is available at GitHub (https://github.com/finalbeibeiyu/Nature_aging).
References
Prince, M. J. et al. The burden of disease in older people and implications for health policy and practice. Lancet 385, 549–562 (2015).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Brunet, A., Goodell, M. A. & Rando, T. A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 24, 45–62 (2023).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Sanz-Ros, J. et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 8, eabq2226 (2022).
Mehdipour, M. et al. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging 12, 8790–8819 (2020).
Castellano, J. M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017).
Galipeau, J. & Sensébé, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833 (2018).
Zhang, K. & Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 1, 608–609 (2023).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Li, X. H. et al. Physiological and pathological insights into exosomes in the brain. Zool. Res. 41, 365–372 (2020).
Chen, X. et al. Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism. Nat. Aging 4, 814–838 (2024).
Lei, J. et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell 13, 220–226 (2022).
Hoang, D. M. et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 7, 272 (2022).
Han, Y. et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 7, 92 (2022).
Qin, T. et al. A population of stem cells with strong regenerative potential discovered in deer antlers. Science 379, 840–847 (2023).
Landete-Castillejos, T. et al. Antlers - evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone 128, 115046 (2019).
Sharpless, N. E. & DePinho, R. A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 8, 703–713 (2007).
Heezen, L. G. M. et al. Spatial transcriptomics reveal markers of histopathological changes in Duchenne muscular dystrophy mouse models. Nat. Commun. 14, 4909 (2023).
Luo, W. D. et al. Age-related self-DNA accumulation may accelerate arthritis in rats and in human rheumatoid arthritis. Nat. Commun. 14, 4394 (2023).
Yin, A. et al. Exercise-derived peptide protects against pathological cardiac remodeling. eBioMedicine 82, 104164 (2022).
Zhou, Y. et al. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen. Med. 6, 34 (2021).
Saul, D. et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 13, 4827 (2022).
Khosla, S. Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1226–1235 (2013).
Wang, L., You, X., Zhang, L., Zhang, C. & Zou, W. Mechanical regulation of bone remodeling. Bone Res. 10, 16 (2022).
Petkovich, D. A. et al. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 25, 954–960.e6 (2017).
Yang, L. et al. Extracellular vesicle-mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation 142, 556–574 (2020).
Zhou, R. et al. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 9, 25 (2021).
Cassady, K., Ruitenberg, M. F. L., Reuter-Lorenz, P. A., Tommerdahl, M. & Seidler, R. D. Neural dedifferentiation across the lifespan in the motor and somatosensory systems. Cereb. Cortex 30, 3704–3716 (2020).
Huang, K. et al. A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat. Commun. 12, 2784 (2021).
Brinkman, J. & Kuypers, H. G. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain 96, 653–674 (1973).
Chiou, K. et al. Rhesus macaques as a tractable physiological model of human ageing. Phil. Trans. R. Soc. B 375, 20190612 (2020).
Tian, X. G. et al. Inflammation-mediated age-dependent effects of casein kinase 2-interacting protein-1 on osteogenesis in mesenchymal stem cells. Chin. Med. J. 133, 1935–1942 (2020).
Li, C. J. et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metab. 33, 1957–1973.e6 (2021).
Zatorre, R. J., Fields, R. D. & Johansen-Berg, H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536 (2012).
Wagstyl, K., Ronan, L., Goodyer, I. M. & Fletcher, P. C. Cortical thickness gradients in structural hierarchies. Neuroimage 111, 241–250 (2015).
Goto, M. et al. Advantages of using both voxel- and surface-based morphometry in cortical morphology analysis: a review of various applications. Magn. Reson. Med. Sci. 21, 41–57 (2022).
Koenig, S. et al. Age dependent hypothalamic and pituitary responses to novel environment stress or lipopolysaccharide in rats. Front. Behav. Neurosci. 12, 55 (2018).
Bian, Z. et al. Effects of species-relevant auditory stimuli on stress in cats exposed to novel environment. J. Appl. Anim. Welf. Sci. 28, 318–327 (2023).
Kong, D. et al. PKA regulatory IIα subunit is essential for PGD2-mediated resolution of inflammation. J. Exp. Med. 213, 2209–2226 (2016).
Huang, R. et al. Circ_0000811 acts as a miR-15b sponge and inhibits Prkar2a-mediated JAK2/STAT1 pathway to attenuate cerebral ischemic vertigo. Cell Death Discov. 8, 247 (2022).
Rao, S. et al. Extracellular vesicles from human urine-derived stem cells delay aging through the transfer of PLAU and TIMP1. Acta Pharm. Sin. B 14, 1166–1186 (2024).
Robbins, P. D. Extracellular vesicles and aging. Stem Cell Investig. 4, 98 (2017).
Bethlehem, R. A. I. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022).
Yang, Y. et al. Metformin decelerates aging clock in male monkeys. Cell 187, 6358–6378.e6329 (2024).
van Niel, G. et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382 (2022).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023).
Zhou, C. et al. Prospective study comparing [(99m)Tc]Tc-DP-FAPI quantitative SPECT/CT with [(68)Ga]Ga-FAPI-04 PET/CT in patients with gastrointestinal tumors. Mol. Pharm. 21, 5297–5304 (2024).
Beck, D. et al. White matter microstructure across the adult lifespan: a mixed longitudinal and cross-sectional study using advanced diffusion models and brain-age prediction. Neuroimage 224, 117441 (2021).
Shamir, I. & Assaf, Y. Tutorial: a guide to diffusion MRI and structural connectomics. Nat. Protoc. 20, 317–335 (2025).
Cagol, A. et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 79, 682–692 (2022).
Wang, S., Su, Q., Qin, W., Yu, C. & Liang, M. Both fine-grained and coarse-grained spatial patterns of neural activity measured by functional MRI show preferential encoding of pain in the human brain. Neuroimage 272, 120049 (2023).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Acknowledgements
We thank all members of the Huang, Qiu and Yao laboratories for discussions. We thank R. Zhao (Fourth Military Medical University, FMMU) for his assistance with MRI data processing and analysis. We thank C. Yu, D. Si and Z. Gong at the Analysis & Testing Laboratory for Life Sciences and Medicine of FMMU for their assistance with NTA analysis of EVs. We thank F. Kang and J. Ye (Department of Nuclear Medicine, Xijing Hospital) for their assistance of SPECT/CT. We thank S. Zhao and Y. Li (FMMU) for the scanning transmission and electron microscope technical assistance. We thank J. Hu (FMMU) for flow cytometry technical assistance. We thank Z. Hu (KIZ) for raising rhesus macaques and access to the monkey facility. We thank S. Dong, H. Zhu, Y. Zhang, Y. Li and M. Yuan (KIZ) for conducting a behavior assessment of rhesus macaques. We thank X. Zhu (KIZ) and N. Liu (State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences) for analyzing MRI data. We thank R. Heller (Section for Computational and RNA Biology, Department of Biology, University of Copenhagen) for reviewing the manuscript and offering valuable suggestions. We thank Shenzhen Acegen Technology Co. for guidance in data analysis and statistics for technical guidance in whole-genome bisulfite sequencing library preparation. We thank Novogene Co. for providing technical support with RNA-seq. We thank OE Biotech for providing technical support with scRNA-seq. The work was supported by the National Key Research and Development Program of China (2024YFA1802500 (J.H.) and 2023YFC2509905 (Z. Luo)); National Natural Science Foundation of China (82122043 (J.H.), 82372404 (J.H.) and 32225009 (Q.Q.)); the Ministry of Science and Technology of China (STI2030-Major Project grant 2021ZD0200900 (Y. Yao); STI2030-Major Projects grants 2022ZD0205100 (J.W.) and 2021ZD0203900 (J.W.)); the Yunnan Department of Science and Technology (202305AH340006 (Y. Yao)); and the New Cornerstone Investigator Program (W.W.).
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Hirotaka Iijima, Andrei Seluanov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of ABPCs and EVsABPCs.
a, Flow cytometry analysis of the cell-cycle phase distribution in A-BMSCs, F-BMSCs, and ABPCs. b–f, Representative images depicting staining for CFU-F (b), EdU (c), p21 (d), γ-H2AX (e) and ALP (f) staining of cells from the three cell types. Scale bar, 5 mm (b), 200 μm (c and d), 100 μm (e) or 3 mm (f). g, Representative morphological images of cells by transmission electron microscopy. Scale bar, 2 mm (top), 200 μm (bottom). h, Diameter distribution of EVs derived from different cell types by NTA. i, Mean diameters of EVs from three distinct cell sources (n = 5). j, Zeta potential of the EVs was determined by tunable resistance pulse sensing (TRPS) (n = 5). Data points are represented by circles. Boxplots display median (center line), IQR (box limits), and 1.5 × IQR (whiskers). k, CD9, CD81, and TSG101 protein levels in EVs from three distinct cell sources were determined by western blotting. l, Mean concentrations of EVs from three distinct cell sources (n = 5). m, PCA of the transcriptomic characteristics in EVsA-BMSCs, EVsF-BMSCs, and EVsABPCs. n,o, Volcano plot illustrated DEGs (n) and DEPs (o) between EVsABPCs and EVsF-BMSCs. p, Network diagram represented the metascape pathways of downregulated DEGs and DEPs in EVsABPCs compared to EVsF-BMSCs. Each node corresponds to a specific biological process, and the size of the node is proportional to the enrichment score. The similarity between pathways is indicated by the thickness of the connecting lines. Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (i and l). Data are presented as mean ± s.d. ****P < 0.0001.
Extended Data Fig. 2 D-BMSCs and EVsD-BMSCs demonstrate similar rejuvenating capabilities compared to F-BMSCs and EVsF-BMSCs.
a, Representative images of EdU staining and the quantitative analysis of EdU-positive cells (n = 5). Scale bar, 200 μm. b, Representative images of SA-β-Gal staining and their quantitative analysis (n = 5). Scale bar, 200 μm. c, Representative images of γ-H2AX staining of cells and their quantitative analysis (n = 5). Scale bar, 100 μm. d–f, Representative images of ARS (d), ALP (e), and Oil Red O (f) staining of cells and their quantitative analysis (n = 5). Scale bar, 3 mm (d and e) or 100 μm (f). g, Morphology of EVs isolated from D-BMSCs (EVsD-BMSCs), Scale bar, 100 nm. h, Diameter distribution of EVsD-BMSCs as revealed by NTA. i, The zeta potential of the EVsD-BMSCs. The data points are represented by circles. Boxplots display median (center line), IQR (box limits), and 1.5 × IQR (whiskers). j, CD9, CD81, and TSG101 protein levels in EVsD-BMSCs by western blotting. k, Mean EVs concentration in different EVs groups (n = 5). l, Representative images of EdU staining of A-BMSCs treated with different EVs and their quantitative analysis (n = 5). Scale bar, 200 μm. m, Representative images of SA-β-Gal staining of A-BMSCs treated with different EVs and their quantitative analysis (n = 5). Scale bar, 200 μm. n, Representative images of γ-H2AX staining of A-BMSCs treated with different EVs and their quantitative analysis (n = 5). Scale bar, 100 μm. o–q, Representative images of ARS (o), ALP (p), and Oil Red O (q) staining of A-BMSCs treated with different EVs and their quantitative analysis (n = 5). Scale bar, 3 mm (o and p) or 100 μm (q). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (a–f and k–q). Data are presented as mean ± s.d. ****P < 0.0001.
Extended Data Fig. 3 Rejuvenation of A-BMSCs after EVsABPCs treatment.
a, Internalization of PKH26-labeled EVsA-BMSCs and EVsF-BMSCs (yellow) within A-BMSCs stained with DAPI (blue) and phalloidin (white), and quantification of PKH26-labeled EVs in A-BMSCs (n = 5). Scale bar, 10 μm. b–d, Representative images of SA-β-Gal (b), ARS (c), and Oil Red O (d) staining in A-BMSCs following EVsABPCs treatment in a dose-dependent manner and their quantitative analysis (n = 5). Scale bar, 200 μm (b), 3 mm (c), or 100 μm (d). e, Flow cytometric analysis was performed to assess the cell-cycle phase in A-BMSCs treated with different EVs. f–l, Representative images of CFU-F (f), EdU (g), γ-H2AX (h), p21 (i), ARS (j), ALP (k), and Oil red O (l) staining in A-BMSCs following different EVs treatment. Scale bar, 5 mm (f), 200 μm (g and i), 100 μm (h and l) or 3 mm (j and k). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (a–d). Data are presented as mean ± s.d. **P < 0.01, ***P < 0. 001, and ****P < 0.0001.
Extended Data Fig. 4 EVsABPCs rejuvenate A-BMSCs in transcriptomic signature and phenotype.
a–d, GSEA analysis revealed the regulation of DNA repair and maintenance (a), epigenetic alterations and telomere attrition (b), SASP (c), and chronic inflammation and stem cell exhaustion (d) in A-BMSCs following different EVs treatments. e, The relative expression levels of Prkar2a in in EVsABPCs treated and EVsABPCs/shPrkar2a treated A-BMSCs by qPCR (n = 5). f, The relative expression levels of Prkar2a in in EVsA-BMSCs treated and EVsA-BMSCs/Prkar2a treated A-BMSCs by qPCR (n = 5). g,h, Representative images of EdU (g) and ARS (h) staining in A-BMSCs treated with EVsABPCs and EVsABPCs/shPrkar2a and their quantitative analysis (n = 5). Scale bar, 200 μm (g) or 3 mm (h). i,j, Representative images of EdU (i) and ARS (j) staining in A-BMSCs treated with EVsA-BMSCs and EVsA-BMSCs/Prkar2a and their quantitative analysis (n = 5). Scale bar, 200 μm (i) or 3 mm (j). Statistical significance was calculated by two-tailed Student’s t-test (e–j). Datr are presented as mean ± s.d. ****P < 0.0001.
Extended Data Fig. 5 EVsABPCs mitigate age-related bone loss in aged mice.
a, Ex vivo fluorescence images and average radiant efficiency of organs at 48 h after tail vein injection of DiR-labeled EVs. From left to right: heart, liver, spleen, lung, kidney (n = 8). Scale bar, 1,000 μm. b, Representative micro-CT images of femoral cortical bone. Scale bar, 150 μm. c, Quantitative analysis of cortical bone parameters, including Ct.V, Ct.Th, Ct.Ar, and Tt.Ar from different groups (n = 8). d, Representative images of the mechanical strength of femurs from mice treated with EVsA-BMSCs or EVsF-BMSCs. e, The concentration of serum CTX-1 (n = 8). f, Representative images of newly formed trabecular bone, labeled with calcein AM (magenta) and alizarin red (white) in mice treated with EVsF-BMSCs and EVsA-BMSCs. Scale bar, 50 μm. g, A schematic diagram of immunohistochemical staining (ALP, OCN and TRAP staining) in femurs. h–j. Representative images of ALP (h), OCN (i), and TRAP (j) staining in femurs from mice treated with EVs, with the ALP-, OCN-, or TRAP-positive cells presented by orange arrow. Scale bar, 100 μm. k–m, Representative micro-CT reconstruction images (k) and quantitative analysis of the trabecular (l) and cortical bone (m) from mice treated with EVsABPCs or EVsABPCs/shPrkar2a (n = 8). Scale bar, 1,500 μm. n–p, Representative micro-CT reconstruction images (n) and quantitative analysis of the trabecular (o) and cortical bone (p) in mice treated with EVsA-BMSCs or EVsA-BMSCs/Prkar2a (n = 8). Scale bar, 1,500 μm. Statistical significance was determined by one-way ANOVA with Bonferroni’s multiple comparisons test (a, c, and e), two-tailed Student’s t-test (l, m, o, and p in addition to the third plot), or nonparametric Mann–Whitney test (the third plot of p). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Extended Data Fig. 6 The effects of EVsABPCs mitigate organ senescence in aged mice.
a,b, Representative images of Masson’s trichrome staining for liver (a) and kidney (b). Scale bar,100 μm (top of a and b) or 350 μm (bottom of a and b). c, Representative images of immunofluorescence staining of TUNEL in the kidney. Scale bar, 200 μm. d,e, Representative images of H&E (d) and Masson’s trichrome (e) staining for skin. Scale bar, 50 μm (d) or 100 μm (e). f, Representative immunofluorescence images of γ-H2AX in skin. Scale bar, 500 μm. g, Representative immunofluorescence images of γ-H2AX staining in brain. Scale bar, 100 μm. h,i, The percentage of collagen fiber area in liver (h) and kidney (i) measured by the Masson’s trichrome staining (n = 8). j, Quantitative analysis of expression level of TUNEL in kidney (n = 8). k–n, Quantitative analysis of epidermal thickness (k) and number of epidermal cells per millimeter thickness (l) measured by H&E staining, as well as dermal thickness (m) and collagen content (n) measured by the Masson’s trichrome staining (n = 8). o, Quantitative analysis of expression level of γ-H2AX in skin (n = 8). p, Quantitative analysis of expression level of γ-H2AX in brain (n = 8). q, Clustering trend plots showed the numbers of DEGs across different organs. r, Cluster analysis showed the main upregulated DEGs in liver, kidney, skin, intestine, brain and pan-tissues treated with EVsABPCs. s–u, Representative tracking images of Y maze (s), NOR (t), and EPM (u) in EVsA-BMSCs and EVsF-BMSCs groups. v, Ex vivo fluorescence images of brains at 48 h after injection of DiR-labeled EVs by tail vein and their quantitative analysis (n = 8). Scale bar, 500 μm. Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (h–p and v). Data were presented as mean ± s.d. ***P < 0.001, and ****P < 0.0001.
Extended Data Fig. 7 EVsABPCs rescue multiple age-associated phenotypes in aged female mice.
a, Representative Micro-CT reconstruction images of femurs with different EVs treatment from female mice. Scale bar, 1,500 μm (left) or 300 μm (right). b, Quantitative analysis of trabecular bone from different groups, including BMD, BV/TV, Tb.Th, Tb.N, SMI, and Tb.Sp (n = 8). c,d, Representative images of the mechanical strength of femurs in female mice (c). Quantitative analysis of the maximum load, yield load, and Young’s modulus (n = 8) (d). e,f, Representative images (e) and quantitative analysis (f) of MAR and BFR/BS in female mice from different groups (n = 8). Scale bar, 50 μm. g, The serum concentration of OCN and P1NP in female mice from different groups (n = 8). h, Quantification of the changes in motor coordination (left) and fatigue resistance (right) tests in female mice (n = 8). i, The serum concentration of inflammatory factors (IL-8, IL-6, IL-1β and TNF) levels with EVs treatment (n = 8). j, Representative SA-β-gal staining and quantification for SA-β-Gal activity in liver, kidney, skin, and brain (from top to bottom) of aged female mice with EVs treatment (n = 8). Scale bar, 50 μm (liver, skin, and brain) or 200 μm (kidney). k, Spatial working memory was assessed using the Y maze as the discrimination index for the novel arm (n = 8). l, Object recognition memory was assessed by NOR as the percentage of time exploring the novel object (n = 8). m, Anxiety-like behavior was assessed by EPM as the percentage of open arm (n = 8). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (b, d, and f–m). Data are presented as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Extended Data Fig. 8 EVsABPCs ameliorate bone loss and improved locomotor functions in aged rhesus macaques.
a, Representative three-dimensional CT reconstructed images of cortical bone, with regions of interest colored in purple. Scale bar, 2,500 μm. b, Quantitative analysis of cortical bone in aged rhesus macaques following EVs treatments, including Ct.V, Ct.Th, Ct.Ar, and Tt.Ar (n = 3). c–f, Quantification of exercise model, including steps per minute (c), as well as sleep model, including total sleep time (d), wake after sleep onset (e), and number of awakenings (f) in aged rhesus macaques treated with EVs during the noninvasive monitor neck collar test. Data was collected daily over a consecutive 7-day recording period (n = 3). Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (b, c, and f), or nonparametric Kruskal–Wallis test with Dunn’s multiple comparisons test (d and e). Data were presented as mean ± s.d. ***P < 0.001.
Extended Data Fig. 9 Indicators of blood examination and bone marrow scRNA-seq in aged rhesus macaques.
a, The counts of white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), and platelets (PLT) in the blood, along with serum levels of creatine kinase (CK), blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), globulin (GLO), albumin-to-globulin ratio (A/G), glucose (GLU), total cholesterol (CHOL), triglycerides (TRIG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and creatinine (CRE2) were measured (n = 3). b, t-SNE plots were generated to depict the expression of specific genes representing seven cell populations: dendritic cells (LILRA4), T-cells (CD3E), plasma cells (MZB1), B-cells (CD79A), erythroid cells (HBA1), granulocytes (MPO), and monocytes (VCAN). c, A combined two-dimensional visualization of single-cell clusters in aged rhesus macaques with different treatments. d, A violin plot was utilized to display the scores of SASP gene expression across seven immune cell lineages (n = 3). Violin plots depict the data distribution (kernel density), density (violin width), IQR (embedded box), mean (white dot), and mean ± s.d. (whiskers). Lines connecting the medians across groups illustrate the overall trend of the data distribution. e, GSEA showed the regulated pathways in the monocyte subpopulation when comparing the EVsF-BMSCs group to the vehicle group. Statistical significance was calculated by one-way ANOVA with Bonferroni’s multiple comparisons test (a and d). Data were presented as mean ± s.d. **P < 0.01, and ****P < 0.0001.
Extended Data Fig. 10 Changes related to brain aging in EVsABPCs-treated aged rhesus macaques.
a, A quantitative analysis was performed to evaluate changes in cortical thickness and surface area following EVs treatment (n = 3). b, VBM analysis showed region variations in GMV among groups, with red areas indicating significant differences in GMV. Quantitative analysis of the significantly different brain regions in GMV following different treatments (n = 3). c, The comparison of GMV between pre-treatment and post-treatment at an uncorrected P-value threshold was performed specifically for the EVsABPCs group. Quantification of GMV changes between pre-treatment and post-treatment in the EVsABPCs group was conducted without correction for multiple comparisons (n = 3). The lines connect the same individuals, showing the trend between pre- and post-treatment. d, A quantitative analysis of the changes of FA and RD following EVs treatment (n = 3). Statistical analyses were performed using the Kruskal–Wallis nonparametric test followed by post hoc pairwise comparisons followed by one-tailed Mann–Whitney U-tests with FWE correction (a and d), or not (b); one-tailed Wilcoxon matched-pairs signed rank test for within-group comparisons (c). Data were presented as mean ± s.d. Uncorrected *P < 0.05.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Supplementary Tables 1–8.
Supplementary Data 1
Volumetric brain maps for percentage changes in GMV and WMV from baseline.
Supplementary Data 2
Statistical source data of supplementary figures.
Supplementary Data 3
Source data of DNA methylation.
Supplementary Video 1
Video footage recorded by four cameras in 3D fine-scale primate behavior tests, camera 1.
Supplementary Video 2
Video footage recorded by four cameras in 3D fine-scale primate behavior tests, camera 2.
Supplementary Video 3
Video footage recorded by four cameras in 3D fine-scale primate behavior tests, camera 3.
Supplementary Video 4
Video footage recorded by four cameras in 3D fine-scale primate behavior tests, camera 4.
Source data
Source Data Fig. 1
Statistical source data of Fig. 1.
Source Data Fig. 2
Statistical source data of Fig. 2.
Source Data Fig. 3
Statistical source data of Fig. 3.
Source Data Fig. 4
Statistical source data of Fig. 4.
Source Data Fig. 5
Statistical source data of Fig. 5.
Source Data Fig. 6
Statistical source data of Fig. 6.
Source Data Fig. 7
Statistical source data of Fig. 7.
Source Data Fig. 8
Statistical source data of Fig. 8.
Source Data Extended Data Fig. 1
Statistical source data of Extended Data Fig. 1 with unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data of Extended Data Fig. 2 with unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data of Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data of Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data of Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data of Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data of Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Statistical source data of Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Statistical source data of Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Statistical source data of Extended Data Fig. 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hao, Y., Yu, B., Qin, M. et al. Extracellular vesicles from antler blastema progenitor cells reverse bone loss and mitigate aging-related phenotypes in mice and macaques. Nat Aging (2025). https://doi.org/10.1038/s43587-025-00918-x